
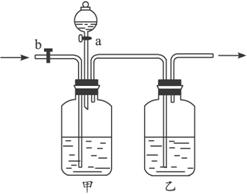
(1)��ƿ��Ӧװ����Լ���__________��Һ����ƿ��Ӧװ����Լ���__________��Һ����Һ©����Ӧװ�����__________��Һ��
(2)��CO��CO2�Ļ������ͨ����װ��ʱ���ȷ���õ�����__________������������__________��Ȼ��õ�����__________������������__________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
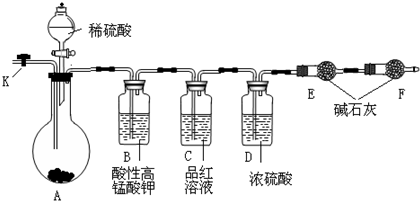
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ��һ��ѧ�ڵ�һѧ�ο��Ի�ѧ�Ծ� ���ͣ�ʵ����
ijУ��ѧ�о���ѧϰС���ͬѧ��ѧϰ�˰������ʺ����ۣ�������ȵ�˼�룬��Ȼ�������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ��������ʵ����ȡ������̽���������⡣��������С��Ļ����������о���
��һ����ȡ����
��1��д��ʵ����ȡ�����Ļ�ѧ����ʽ������������������������������ ������
��2����ͬѧģ���ű���ʳ��ˮ�ռ������ķ����������ű����Ȼ����Һ�ķ����ռ�����������Ϊ���ܷ�ﵽĿ�ģ������������������� ����ܡ���������������������������������������������������������
��������С����ijͬѧ���������ͼ��ʾ��ʵ��װ�ã����ּгּ�β������װ��δ��������̽�������Ļ�ԭ�ԣ�
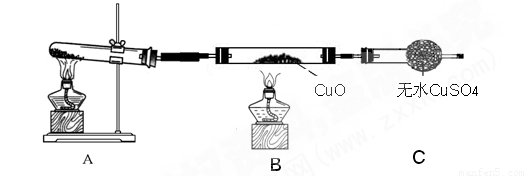 ������3����װ�����������һ��ȱ�ݣ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ�������������������������������������� ���� ��
������3����װ�����������һ��ȱ�ݣ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ�������������������������������������� ���� ��
��4�����øĽ����װ�ý���ʵ�飬�۲쵽CuO��Ϊ��ɫ���ʣ���ˮCuSO4����ɫ��ͬʱ����һ������Ⱦ�����塣д��������CuO��Ӧ�Ļ�ѧ����ʽ������������������������������������������������
��������������
�� ��5����ͬѧ��Ϊ��NH3��CuO��Ӧ���ɵĺ�ɫ���ʿ��ܺ���Cu2O����֪��Cu2O��һ�ּ����������������Һ�У�Cu+ ���ȶ��Ա�Cu2+ �Cu+ Cu+Cu2+�����������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O��������������������������������������
������
Cu+Cu2+�����������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O��������������������������������������
������
��6����������⣨5������ͬѧ�����Cu2O��CuO�����ȶ����ĸ���ǿ���������ǽ���������ʵ�飺ȡ98 g Cu(OH)2���壬������80��~100��ʱ���õ���ɫ�����ĩ���������ȵ�1000�����ϣ���ɫ��ĩȫ����Ϊ��ɫ��ĩA����ȴ�������A������Ϊ72 g���ݴ˿��Ƶã�A�Ļ�ѧʽΪ������������ ���ɴˣ��õ��Ľ�����________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)��ƿ��Ӧװ����_______��Һ����ƿ��Ӧװ����_______��Һ����Һ©����Ӧװ����_______��Һ��
(2)��CO��CO2�Ļ������ͨ����װ��ʱ���ȷ���õ�����_______������������_______��Ȼ��õ�����_______������������_______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com