
�Ӻ�ͭ��������Ͳ��Ŀ�״������������ȡ����������һ�ֹ������£�
[��Դ:ѧ#��#��Z#X#X#K]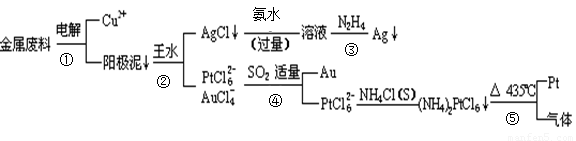
�������Ϲ��ջش��������⣺
��1�����ʱ����_____________Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ���������ĵ缫��Ӧ����ʽΪ
��2��AgCl���ڰ�ˮ�����õ���Һ���е�һ�������ӣ��ڼ��������£�Ҳ���������ǽ��仹ԭΪ����д���÷�Ӧ�����ӷ���ʽ��
��3��д������ܵ����ӷ���ʽ��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Au +6HNO3(Ũ)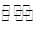 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
��5��д����Ӧ�ݵĻ�ѧ��Ӧ����ʽ
____________________________________________________________________________
����15�֣�
��1���������ϣ�1�֣���Cu2+ + 2e �� Cu��2�֣�
�� Cu��2�֣�
��2��2Ag(NH3)2+
+ 2OH��+CH2OH(CHOH)4CHO��CH2OH(CHOH)4COO + NH4+ + 2Ag��+ 3NH3 + H2O��ˮԡ���ȣ���3�֣�
+ NH4+ + 2Ag��+ 3NH3 + H2O��ˮԡ���ȣ���3�֣�
��3��2AuCl4 + 3SO2 + 6 H2O = 2Au + 8Cl
+ 3SO2 + 6 H2O = 2Au + 8Cl + 3SO42
+ 3SO42 + 12H+ ��3�֣�
+ 12H+ ��3�֣�
��4����ˮ�к��д�����Cl ��Au3+��Cl��������AuCl4
��Au3+��Cl��������AuCl4 ��ʹ��ƽ����Au3+Ũ�Ƚ��ͣ�ƽ�����ƣ���������ˮ����3�֣�
��ʹ��ƽ����Au3+Ũ�Ƚ��ͣ�ƽ�����ƣ���������ˮ����3�֣�
��5��3(NH4)2 PtCl6 == 3Pt + 2N2 +18HCl + 2NH3��435�棩��3�֣�
��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ӻ�ͭ��������Ͳ��Ŀ�״������������ȡ����������һ�ֹ������£�
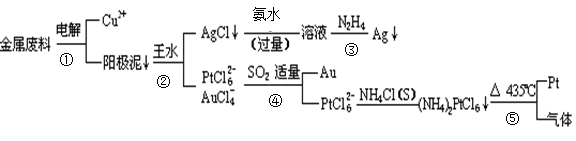
�������Ϲ��ջش��������⣺
��1�����ʱ����_____________Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ���������ĵ缫��Ӧ����ʽΪ
��2��AgCl���ڰ�ˮ�����õ���Һ���е�һ�������ӣ��ڼ��������£�Ҳ���������ǽ��仹ԭΪ����д���÷�Ӧ�����ӷ���ʽ��
��3��д������ܵ����ӷ���ʽ��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Au +6HNO3(Ũ)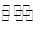 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
��5��д����Ӧ�ݵĻ�ѧ��Ӧ����ʽ
____________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������һ��2010��������ѧ�ڿ�ǰģ�����ۻ�ѧ ���ͣ������
�Ӻ�ͭ��������Ͳ��Ŀ�״������������ȡ����������һ�ֹ������£�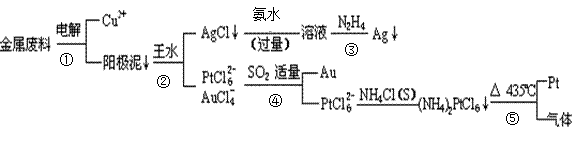
�������Ϲ��ջش��������⣺
��1�����ʱ����_____________Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ���������ĵ缫��Ӧ����ʽΪ
��2��AgCl���ڰ�ˮ�����õ���Һ���е�һ�������ӣ��ڼ��������£�Ҳ���������ǽ��仹ԭΪ����д���÷�Ӧ�����ӷ���ʽ��
��3��д������ܵ����ӷ���ʽ��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Au +6HNO3(Ũ)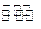 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
��5��д����Ӧ�ݵĻ�ѧ��Ӧ����ʽ
____________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ӻ�ͭ��������Ͳ��Ŀ�״������������ȡ����������һ�ֹ������£�
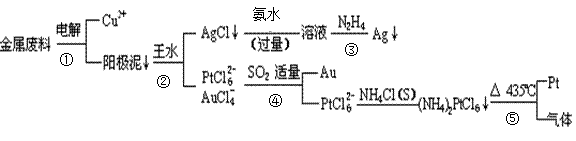
�������Ϲ��ջش��������⣺
��1�����ʱ����_____________Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ���������ĵ缫��Ӧ����ʽΪ
��2��AgCl���ڰ�ˮ�����õ���Һ���е�һ�������ӣ��ڼ��������£�Ҳ���������ǽ��仹ԭΪ����д���÷�Ӧ�����ӷ���ʽ��
��3��д������ܵ����ӷ���ʽ��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Au +6HNO3(Ũ)![]() Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
��5��д����Ӧ�ݵĻ�ѧ��Ӧ����ʽ
____________________________________________________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com