

���� ��ͭ�����ࣨ��Ҫ�ɷ�ΪCu��Cu2S��Cu2Se��Cu2Te�ȣ����뱺�գ��õ�SeO2��SO2��CuO��TeO2�ȣ������������������Һ�к���CuSO4��TeOSO4�ȣ��õ�ⷨ��ȥͭ��ͨ���������TeOSO4�����������������ԭ��Ӧ���ɴ��ڣ�
��1����������������������Cu��Cu2S��Cu2Se��Cu2Te���ɶ�Ӧ�������ͨ���ʹͭ��������ڣ��ɽ��ұ�����ķ���¯ԭ��������Ӧ�Ӵ��������߷�Ӧ���ʣ�
��2��SeO2��SO2�Ļ����������ˮ�����Ƶõ���Se��˵��Se������ԭ��Ӧ����SO2����������Ӧ������������Ϊ+6����ΪH2SO4���ɸ��ݵ����غ�������������뻹ԭ��������ʵ���֮�ȣ�NaHSeO3��Һ�У�����HSeO3-�ĵ����ˮ�⣬�ɽ��Ka1=2.5��10-3��Ka2=2.6��10-7�ж�ˮ��̶������̶ȵ���Դ�С���ж���Һ������ԣ�
��3������ʱ�õ�SeO2��SO2��CuO��TeO2�ȣ������������������Һ�к���CuSO4��TeOSO4�ȣ���ԭʱ��Ҫ���ɴ��ж�SO2��
��4������ԭ��������TeOSO4����ԭ����Te��SO2������Ϊ���ᣬ���ݵ����غ��ԭ���غ�ɵô˷�Ӧ�Ļ�ѧ����ʽ�����ص�����������ԭ��Ӧ���ɽ����Һ�ļ��Ի���д�������缫��Ӧʽ��
��5�����ݷ�Ӧ�ķ���ʽ��֪SeO2��2I2��4Na2S2O3������Ʒ��Se���������ټ����������������ɣ�
��� �⣺��1������������ǿ�����Կ�֪������ʱͨ�������������������ȼ������ͨ������ʹͭ��������ڣ�������ұ�����ķ���¯��������������ͭ������ĽӴ�������ӿ췴Ӧ���ʣ��ʴ�Ϊ������������ȼ������������ͭ������ĽӴ�������ӿ췴Ӧ���ʣ�
��2��SeO2��SO2�Ļ����������ˮ�����Ƶõ���SeΪ��ԭ���ÿ����1molSeת��4mol���ӣ���Ӧͬʱ�õ���H2SO4Ϊ�������ÿ����1mol����ת��2mol���ӣ����ݵ����غ㣬��÷�Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ2��1�������ᣨH2SeO3����Ka1=2.5��10-3��Ka2=2.6��10-7����֪HSeO3-��ˮ�ⳣ��Kh=$\frac{{K}_{w}}{{K}_{{a}_{2}}}$=$\frac{1��1{0}^{-14}}{2.6��1{0}^{-7}}$=0.38��10-7����֪Ka2��Kh������NaHSeO3��Һ�����ԣ�pH��7��
�ʴ�Ϊ��2��1������Ka2=2.6��10-7��Kh=0.38��10-7����֪Ka2��Kh��HSeO3-����̶ȴ���ˮ��̶ȣ���Һ�����ԣ�
��3�����պ�õ�CuO��TeO2���������ᣬ����TeOSO4��ͬʱ����CuSO4���������ɵ�SO2�����ڻ�ԭ����SO2��ѭ�����ã�
�ʴ�Ϊ��CuSO4��SO2��
��4��TeOSO4�����������������ԭ��Ӧ���ɴ��ڣ�����ʽΪ2SO2+TeOSO4+3H2O=Te+3H2SO4��ͨ�����Ի����µ��Na2TeO3��Һ���Te����ʱ�����Ϸ�����ԭ��Ӧ�ĵ缫��ӦʽΪTeO32-+4e-+3H2O=Te+6OH-��
�ʴ�Ϊ��2SO2+TeOSO4+3H2O=Te+3H2SO4��TeO32-+4e-+3H2O=Te+6OH-��
��5�����ݷ�Ӧ�ķ���ʽ��֪SeO2��2I2��4Na2S2O3�����ĵ�n��Na2S2O3��=0.2000 mol/L��0.024L=0.0048mol��
���ݹ�ϵʽ������Ʒ��n��SeO2��=0.0048mol��$\frac{1}{4}$=0.0012mol����Se������Ϊ0.0012mol��79g/mol=0.0948g��
������Ʒ��Se����������Ϊ$\frac{0.0948g}{0.1200g}$��100%=79%��
�ʴ�Ϊ��79%��
���� ���⿼��ʵ���Ʊ�������������ԭ��Ӧ�ζ����㡢���ʵķ����ᴿ��������������ķ������ۣ��Ƕ�ѧ���ۺ������Ŀ��飬ע����Ŀ��Ϣ��Ǩ�����ã���ȷ�Ʊ����̡�������Ӧԭ��Ϊ���ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Aԭ�ӵõ����ӵ���Ŀ��Bԭ���� | |
| B�� | AԪ�ص�������۱�BԪ�ص��������Ҫ�� | |
| C�� | ��̬�⻯������ˮ������ԣ�A��Bǿ | |
| D�� | A��������B���⻯��ˮ��Һ��Ӧ������B���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����75%�ľƾ���ҽ�������� | |
| B�� | �����յķ������Լ���ë֯�����֯�� | |
| C�� | Һ��ʯ��������Ȼ������Ҫ�ɷֶ�Ϊ���� | |
| D�� | ���ع��͡���ֹʳ�ã������������Ʒ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 80��ʱ��1 L pH=1��������Һ�У����е�OH-��ĿΪ10-13NA | |
| B�� | ��0.1molNH4HSO4����Һ�У���������Ŀ��С��0.2NA | |
| C�� | C3H8�����е�2��Hԭ�ӷֱ�1��-NH2��1��-OHȡ����1mol���л����������õ��Ӷ���ĿΪ13NA | |
| D�� | ��Mg��AlΪ�缫��NaOH��ҺΪ�������Һ��ԭ����У�����������NA�����ӣ��������ų�H2�����Ϊ11.2 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶��C��D��A��B | B�� | ���Ӱ뾶��D��C��A��B | ||
| C�� | ԭ��������B��A��D��C | D�� | �⻯���ȶ��ԣ�D��C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ӣ�S-2 | |
| B�� | �õ���ʽ��ʾ�Ȼ�����ӵ��γɹ��̣� | |
| C�� | �������ĵ���ʽ�� | |
| D�� | HClO�Ľṹʽ��H-O-Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | п��ϡ����ķ�Ӧ | B�� | �Ȼ���������������巴Ӧ | ||
| C�� | ˫��ˮ�ķֽⷴӦ | D�� | ��������������ķ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ˮ�� | B�� | �轺������� | ||
| C�� | Ư�۾��������� | D�� | ������ʳƷ���ڵ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
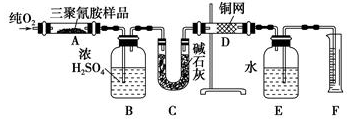
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com