
| A£® | 25”ꏱ£¬HAµÄµēĄėĘ½ŗā³£ŹżKaŌ¼ĪŖ1.43”Į10-3 | |
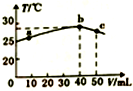
| B£® | a”śbµÄ¹ż³ĢÖŠ£¬ČÜŅŗÖŠc£ØA-£©Óėc£ØHA£©Ö®ŗĶŹ¼ÖÕ²»±ä | |
| C£® | b”ścµÄ¹ż³ĢÖŠ£¬ĪĀ¶Č½µµĶµÄÖ÷ŅŖŌŅņŹĒČÜŅŗÖŠ·¢ÉśĮĖĪüČČ·“Ó¦ | |
| D£® | µČÅØ¶ČµÄNaOHŗĶNaA»ģŗĻČÜŅŗÖŠŅ»¶Ø“ęŌŚ¹ŲĻµ£ŗc£ØNa+£©£¾c£ØA-£©£¾c£ØOH-£©£¾c£ØH+£© |
·ÖĪö A£®µēĄėĘ½ŗā³£ŹżK=$\frac{c£Ø{H}^{+}£©c£Ø{A}^{-}£©}{c£ØHA£©}$¼ĘĖćµĆµ½ÅŠ¶Ļ£»
B£®µ±Ėį¼īÖŠŗĶĒ”ŗĆĶźČ«Ź±£¬ČÜŅŗÖŠµÄČÜÖŹĪŖNaA£¬øł¾ŻĪļĮĻŹŲŗćc£ØA-£©Óėc£ØHA£©Ö®ŗĶŹ¼ÖÕµČÓŚÄĘĄė×ÓÅØ¶Č£¬ČōĖį¹żĮæ»ņ¼ī¹żĮæŹ±²»ŌŁĻąµČ£»
C£®b”ścµÄ¹ż³ĢÖŠ£¬ĪĀ¶Č½µµĶµÄŌŅņŹĒČÜŅŗÖŠ·¢ÉśĮĖ·“Ó¦Ē”ŗĆÉś³ÉNaA£¬¼ĢŠųµĪ¼ÓĒāŃõ»ÆÄĘČÜŅŗ²»ŌŁ·¢Éś·“Ó¦£»
D£®øł¾ŻĪļĮĻŹŲŗć£¬µČÅØ¶ČµÄNaOHŗĶNaA»ģŗĻČÜŅŗÖŠc£ØNa+£©£¾c£ØOH-£©£¾c£ØA-£©£¾c£ØH+£©£®
½ā“š ½ā£ŗA£®µēĄėĘ½ŗā³£ŹżK=$\frac{c£Ø{H}^{+}£©c£Ø{A}^{-}£©}{c£ØHA£©}$=$\frac{0.01mol/L”Į0.01mol/L}{0.08mol/L-0.01mol/L}$=1.43”Į10-3£¬¹ŹAÕżČ·£»
B£®µ±Ėį¼īÖŠŗĶĒ”ŗĆĶźČ«Ź±£¬ČÜŅŗÖŠµÄČÜÖŹĪŖNaA£¬øł¾ŻĪļĮĻŹŲŗćc£ØA-£©+c£ØHA£©=c£ØNa+£©£¬ČōĖį¹żĮæ»ņ¼ī¹żĮæŹ±²»ŌŁĻąµČ£¬¹ŹB“ķĪó£»
C£®b”ścµÄ¹ż³ĢÖŠ£¬ĪĀ¶Č½µµĶµÄŌŅņŹĒČÜŅŗÖŠ·¢ÉśĮĖ·“Ó¦Ē”ŗĆÉś³ÉNaA£¬¼ĢŠųµĪ¼ÓĒāŃõ»ÆÄĘČÜŅŗ²»ŌŁ·¢Éś·“Ó¦£¬ČÜŅŗĪĀ¶Č½µµĶ£¬¹ŹC“ķĪó£»
D£®øł¾ŻĪļĮĻŹŲŗć£¬µČÅØ¶ČµÄNaOHŗĶNaA»ģŗĻČÜŅŗÖŠc£ØNa+£©£¾c£ØOH-£©£¾c£ØA-£©£¾c£ØH+£©£¬¹ŹD“ķĪó£®
¹ŹŃ”A£®
µćĘĄ ±¾Ģāæ¼²éĖį¼ī»ģŗĻµÄ¶ØŠŌÅŠ¶ĻŗĶ¼ĘĖć£¬ĢāÄæ½įŗĻÖŠŗĶ·“Ӧ漲éĮĖĶ¬Ń§ĆĒ¹Ū²ģ·ÖĪöĪŹĢāµÄÄÜĮ¦£¬ŅŌ¼°ĄūÓĆ»Æѧ·½³ĢŹ½¼ĘĖćµÄÄÜĮ¦£¬±Č½Ļ×ŪŗĻ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĖįČÜŅŗµÄµ¼µēŠŌ±ČŃĪĖįČÜŅŗµÄČõ | |
| B£® | 0.1mol/LŅŅĖįÄĘČÜŅŗµÄpHŌ¼ĪŖ8 | |
| C£® | °Ń0.1mol/LŅŅĖįČÜŅŗĪĀ¶ČÉżøß10”ęŗó£Ø²»æ¼ĀĒĖ®Õō·¢£©£¬Ęä pH½µµĶ | |
| D£® | µČÅØ¶ČµČĢå»żµÄŅŅĖįŗĶŃĪĖįŌŚæŖŹ¼ÓėĶ¬Ńł“󊔵ÄĆ¾·“Ó¦Ź±£¬ŃĪĖį·“Ó¦æģ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
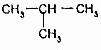 µÄ¹ŲĻµŹĒĶ¬ĻµĪļ
µÄ¹ŲĻµŹĒĶ¬ĻµĪļ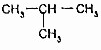 £ØŅģ¶”Ķ飩µÄ¹ŲĻµŹĒĶ¬·ÖŅģ¹¹Ģ壮
£ØŅģ¶”Ķ飩µÄ¹ŲĻµŹĒĶ¬·ÖŅģ¹¹Ģ壮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ĆüĆū | Ņ»ĀČ“śĪļ | |
| A | 2-¼×»ł-2-ŅŅ»ł±ūĶé | 3 |
| B | 1£¬3-¶ž¼×»ł±½ | 3 |
| C | 2£¬2£¬3-Čż¼×»łĪģĶé | 6 |
| D | 2£¬3-¶ž¼×»ł-4-ŅŅ»ł¼ŗĶé | 7 |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
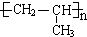 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®“ÓŌ×Ó½į¹¹µÄ½Ē¶Č·ÖĪöB”¢NŗĶOµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņĪŖN£¾O£¾B£®
£®“ÓŌ×Ó½į¹¹µÄ½Ē¶Č·ÖĪöB”¢NŗĶOµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņĪŖN£¾O£¾B£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ČõĖį | CH3COOH | HCN | H2CO3 |
| µēĄėĘ½ŗā³£Źż£Ø25”ę£© | K1=1.8”Į10-5 | K1=4.9”Į10-10 | K1=4.3”Į10-7 K2=5.6”Į10-11 |
| A£® | µČĪļÖŹµÄĮæÅØ¶ČŹ±£ŗpH£ØNa2CO3£©£¾pH£ØNaCN£©£¾pH£ØNaHCO3£©£¾pH£ØCH3COONa£© | |
| B£® | ÖŠŗĶµČĢå»ż”¢µČpHµÄCH3COOHČÜŅŗŗĶHCNČÜŅŗĻūŗÄNaOHµÄĮæĒ°Õß“óÓŚŗóÕß | |
| C£® | ĻņNaCNÖŠĶØČėÉŁĮæµÄCO2£ŗCN -+H2O+CO2=HCO3 -+HCN | |
| D£® | 0.2mol/L HCNÓė0.1mol/L NaOHČÜŅŗµČĢå»ż»ģŗĻŗóĻŌ¼īŠŌ£¬Ōņc£ØNa+£©£¾c£ØCN-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | »Æѧ·“Ó¦ | ²āĮæŅĄ¾Ż£Øµ„Ī»Ź±¼äÄŚ£© |
| A | CO£Øg£©+H2O£Øg£©=CO2£Øg£©+H2£Øg£© | Ń¹Ēæ±ä»Æ |
| B | Zn+H2SO4=ZnSO4+H2 | H2Ģå»ż |
| C | 2NO2?N2O4 | ŃÕÉ«ÉīĒ³ |
| D | Ca£ØOH£©2+Na2CO3=CaCO3”ż+2NaOH | ³ĮµķÖŹĮæ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com