
½üÄźĄ“ŌĖÓĆÓŠ»śŗĻ³ÉµÄ·½·ØÖʱø³öĮĖŠķ¶ą¾ßÓŠĻĀĶ¼ĖłŹ¾Į¢Ģå½į¹¹µÄ»·×“»ÆŗĻĪļ£¬Čē
£Ø1£©Š“³öÉĻŹöĪļÖŹµÄ·Ö×ÓŹ½£ŗ¢ń__________£¬¢ņ__________£¬¢ó________”£
£Ø2£©¢ń”¢¢ņ”¢¢óÖ®¼äµÄ¹ŲĻµŹĒ________”£
A£®Ķ¬ĖŲŅģŠĪĢå B£®Ķ¬ĻµĪļ
C£®Ķ¬·ÖŅģ¹¹Ģå D£®Ķ¬ÖÖĪļÖŹ
£Ø3£©ĄūÓĆ½į¹¹¢ńÄܽāŹĶ±½µÄĻĀĮŠŹĀŹµµÄŹĒ____£¬ĄūÓĆ½į¹¹¢ņ»ņ¢ó²»ÄܽāŹĶ±½µÄĻĀĮŠŹĀŹµµÄŹĒ______”£
A£®±½²»ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ«
B£®±½²»ÄÜÓėäåĖ®Ņņ·¢Éś»Æѧ·“Ó¦¶ųŹ¹äåĖ®ĶŹÉ«
C£®ŌŚŅ»¶ØĢõ¼žĻĀÄÜŗĶH2·¢Éś¼Ó³É·“Ó¦
 °Ł·Öѧɜ×÷Ņµ±¾ĢāĮ·ĶõĻµĮŠ“š°ø
°Ł·Öѧɜ×÷Ņµ±¾ĢāĮ·ĶõĻµĮŠ“š°ø »„¶ÆæĪĢĆĻµĮŠ“š°ø
»„¶ÆæĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ¹ŲÓŚ¼×ĶéµÄĖµ·Ø²»ÕżČ·µÄŹĒ( )
| A£®¼×Ķé·Ö×Ó¾ßÓŠÕżĖÄĆęĢå½į¹¹ |
| B£®¼×Ķé·Ö×ÓÖŠµÄĖÄøöC”ŖH¼üŹĒĶźČ«µČ¼ŪµÄ |
| C£®¼×Ķé·Ö×ÓŹĒĘ½ĆęÕż·½ŠĪ½į¹¹ |
D£®¼×Ķé·Ö×ӵĽį¹¹Ź½ŹĒ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijĢžA 0£®2 mol ŌŚŃõĘųÖŠĶźČ«Č¼ÉÕŗó£¬Éś³ÉCO2”¢H2Oø÷1£®2 mol”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĢžAµÄ·Ö×ÓŹ½ĪŖ ”£
£Ø2£©ČōČ”Ņ»¶ØĮæµÄĢžAĶźČ«Č¼ÉÕŗó£¬Éś³ÉCO2ŗĶH2Oø÷3 mol£¬ŌņÓŠ gĢžA²Ī¼ÓĮĖ·“Ó¦£¬Č¼ÉÕŹ±Ļūŗıź×¼×“æöĻĀµÄŃõĘų L”£
£Ø3£©ČōĢžA²»ÄÜŹ¹äåĖ®ĶŹÉ«£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀÄÜÓėĀČĘų·¢ÉśČ”“ś·“Ó¦£¬ĘäŅ»ĀČČ”“śĪļÖ»ÓŠŅ»ÖÖ£¬ŌņĢžAµÄ½į¹¹¼ņŹ½ĪŖ ”£
£Ø4£©ČōĢžAÄÜŹ¹äåĖ®ĶŹÉ«£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀ£¬ÓėH2¼Ó³É£¬Ęä¼Ó³É²śĪļ¾²ā¶Ø·Ö×ÓÖŠŗ¬ÓŠ4øö¼×»ł£¬ŌņĢžAæÉÄܵĽį¹¹¼ņŹ½ÓŠ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖĻĀĮŠĪļÖŹ£ŗ

Ēė°“ŅŖĒóĢīæÕ£ŗ
(1)Š“³ö¢Ł¢ÜµÄĆū³Ę________”¢________£»
(2)Š“³ö¢Ś”¢¢ŻµÄ·Ö×ÓŹ½________”¢________£»
(3)»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄŹĒ________”£
(4)Š“³ö¢ŪÓėµČĪļÖŹµÄĮæµÄBr2·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ėę×ÅČ«ĒņŠŌŹÆÓĶĪ£»śµÄĄ“ĮŁ£¬Ź¹Šķ¶ą¹ś¼Ņ¹Ų×¢ÓĆĆŗ“śĢęŹÆÓĶµÄŃŠ¾æ£¬æĘѧ¼Ņ·¢ĻÖŌŚ443”«473 KµÄĪĀ¶ČĻĀ£¬ÓĆCo×÷“߻ƼĮ£¬Ė®ĆŗĘų(Ö÷ŅŖ³É·ÖŹĒCOŗĶH2)æÉŅŌÉś³ÉĢ¼Ō×ÓŹż5”«8Ö®¼äµÄĶéĢž£¬ÕāŹĒÓĆĆŗŗĻ³ÉĘūÓĶµÄ·½·ØÖ®Ņ»”£
(1)ÓĆĆŗŗĻ³ÉĘūÓĶCnH2n£«2µÄÓŠ¹Ų·“Ó¦·½³ĢŹ½ŹĒ
_________________________________________________________________£»
(2)ŅŖ“ļµ½ŗĻ³ÉÉĻŹöĘūÓĶµÄŅŖĒó£¬COŗĶH2µÄĢå»ż±ČµÄȔֵ·¶Ī§ŹĒ”””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijæĪĶā»ī¶ÆŠ”×éĄūÓĆČēĶ¼ĖłŹ¾×°ÖĆĢ½¾æ¼×ĶéÓėĀČĘųµÄ·“Ó¦”£øł¾ŻĢāŅā£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)CH4ÓėCl2·¢Éś·“Ó¦µÄĢõ¼žŹĒ________£»ČōÓĆČÕ¹āÖ±É䣬æÉÄÜ»įŅżĘš_______________________________”£
(2)ŹµŃéÖŠæɹŪ²ģµÄŹµŃéĻÖĻóÓŠ£ŗĮæĶ²ÄŚ±Ś³öĻÖÓĶדŅŗµĪ£¬±„ŗĶŹ³ŃĪĖ®ÖŠÓŠÉŁĮæ¹ĢĢåĪö³ö£¬__________£¬____________________µČ”£
(3)ŹµŃéÖŠÉś³ÉµÄÓĶדŅŗµĪµÄ»ÆѧŹ½ĪŖ________£¬ĘäÖŠ________ŹĒ¹¤ŅµÉĻÖŲŅŖµÄČܼĮ”£
(4)ÓƱ„ŗĶŹ³ŃĪĖ®¶ų²»ÓĆĖ®µÄŌŅņŹĒ____________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĖłŹ¾£¬UŠĪ¹ÜµÄ×ó¶Ė±»Ė®ŗĶ½ŗČū·ā±ÕÓŠ¼×ĶéŗĶĀČĘų£ØĢå»ż±ČĪŖ1”Ć4£©µÄ»ģŗĻĘųĢ壬¼Ł¶ØĀČĘųŌŚĖ®ÖŠµÄČܽā¶ČæÉŅŌŗöĀŌ”£½«·ā±ÕÓŠ¼×ĶéŗĶĀČĘų»ģŗĻĘųĢåµÄ×°ÖĆ·ÅÖĆŌŚÓŠ¹āĮĮµÄµŲ·½£¬ČĆ»ģŗĻĘųĢå»ŗĀżµŲ·“Ó¦Ņ»¶ĪŹ±¼ä”£
£Ø1£©¼ŁÉč¼×ĶéÓėĀČĘų·“Ó¦³ä·Ö£¬ĒŅÖ»²śÉśŅ»ÖÖÓŠ»śĪļ£¬ĒėŠ“³ö»Æѧ·½³ĢŹ½£ŗ______________________________________________”£
£Ø2£©ČōĢāÄæÖŠ¼×ĶéÓėĀČĘųµÄĢå»żÖ®±ČĪŖ1”Ć1£¬ŌņµĆµ½µÄ²śĪļĪŖ____£ØĢī×ÖÄø±ąŗÅ£©”£
A£®CH3Cl””HCl
B£®CCl4””HCl
C£®CH3Cl””CH2Cl2
D£®CH3Cl””CH2Cl2””CHCl3””CCl4””HCl
£Ø3£©¾¹ż¼øøöŠ”Ź±µÄ·“Ó¦ŗó£¬UŠĪ¹ÜÓŅ¶ĖµÄĖ®Öł±ä»ÆŹĒ________
£ØĢī×ÖÄø±ąŗÅ£©”£
A£®Éżøß B£®½µµĶ C£®²»±ä D£®ĪŽ·ØČ·¶Ø
£Ø4£©ČōĖ®ÖŠŗ¬ÓŠNa2SiO3£¬ŌņŌŚUŠĪ¹Ü×ó¶Ė»į¹Ū²ģµ½________________________________”£
£Ø5£©ÓŅ¶Ė²£Į§¹ÜµÄ×÷ÓĆŹĒ_______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĆŗŹĒÖŲŅŖµÄÄÜŌ“£¬Ņ²ŹĒ»Æ¹¤Éś²śµÄÖŲŅŖŌĮĻ”£
£Ø1£©ĆŗČ¼ÉÕ²śÉśµÄ·ĻĘųÖ±½ÓÅŷŵ½æÕĘųÖŠ£¬æÉÄܵ¼ÖĀµÄ»·¾³ĪŪČ¾ĪŹĢāŹĒ_____________”£
£Ø2£©ĻĀĶ¼ŹĒ¶ŌĆŗæóČ¼ÉÕ²śÉśµÄ·ĻĘų½ųŠŠ³£ĪĀĶŃĮņ“¦ĄķµÄĮ÷³ĢŹ¾ŅāĶ¼”£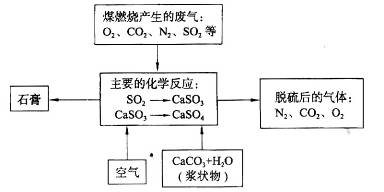
¢Ł·ĻĘųĶŃĮņ¹ż³ĢÖŠ£¬Ö÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________”¢_____________£»
¢ŚŌŚĆŗæóÖŠÖ±½ÓĢķ¼ÓŅ»ÖÖĪļÖŹ£¬æÉÓŠŠ§¼õÉŁĆŗæóČ¼ÉÕ²śÉśµÄSO2£¬øĆĪļÖŹŹĒ___________£»
¢ŪŹÆøąµÄ¹¤ŅµÉś²śÖŠµÄÓĆĶ¾ŹĒ_________________£ØŠ“³öŅ»ÖÖÓĆĶ¾¼“æÉ£©”£
£Ø3£©Ćŗ¾¹ż___________£ØĢī¼Ó¹¤·½·Ø£©æÉŅŌµĆµ½½¹ĀÆĆŗĘų”¢Ćŗæó½¹ÓĶŗĶ½¹Ģ攣Ćŗ½¹ÓĶ¾¹ż_______£ØĢī¼Ó¹¤·½·Ø£©æɵƵ½·¼µĆ×å»ÆŗĻĪļ”£ĆŗæóŅ²æÉŅŌÓĆĒā»Æ·Ø×Ŗ»ÆČ¼ÓĶ£¬Ēā»Æ·ØµÄ±¾ÖŹŹĒ__________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¢ń”¢µĀ¹ś»Æѧ¼ŅææāĄÕČĻĪŖ±½·Ö×ӵĽį¹¹ÖŠ£¬Ģ¼Ģ¼¼äŅŌµ„”¢Ė«¼ü½»Ģę½įŗĻ¶ų³É»·×“”£ĪŖĮĖĘĄ¼ŪææāĄÕµÄ¹Ūµć£¬Ä³Ń§ÉśÉč¼ĘĮĖŅŌĻĀŹµŃé·½°ø£ŗ¢Ł°“ĻĀĶ¼ĖłŹ¾µÄ×°ÖĆĶ¼Į¬½ÓŗĆø÷ŅĒĘ÷£»¢Ś¼ģŃé×°ÖƵÄĘųĆÜŠŌ£»¢ŪŌŚAÖŠ¼ÓČėŹŹĮæµÄ±½ŗĶŅŗäåµÄ»ģŗĻŅŗĢ壬ŌŁ¼ÓČėÉŁĮæĢś·Ū£¬ČūÉĻĻšĘ¤Čū£¬“ņæŖK1”¢K2”¢K3Ö¹Ė®¼Š£»¢Ü“żÉÕĘæCÖŠĘųĢåŹÕ¼ÆĀśŗ󣬽«µ¼¹ÜDµÄĻĀ¶Ė²åČėÉÕ±ĄļµÄĖ®ÖŠ£¬¹Ų±ÕK2£¬“ņæŖK3,¼·Ń¹Ō¤ĻČ×°ÓŠĖ®µÄ½ŗĶ·µĪ¹ÜµÄ½ŗĶ·£¬¹Ū²ģŹµŃéĻÖĻó”£
ŹŌ»Ų“š£ŗ
£Ø1£©Š“³öAÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»ÄÜÖ¤Ć÷ææāĄÕ¹Ūµć“ķĪóµÄŹµŃéĻÖĻóŹĒ £»
£Ø2£©×°ÖĆBµÄ×÷ÓĆŹĒ £»
¢ņ”¢ÓĆÖŠŗĶµĪ¶Ø·Ø²ā¶ØÉÕ¼īµÄ“æ¶Č£¬ČōÉÕ¼īÖŠ²»ŗ¬ÓŠÓėĖį·“Ó¦µÄŌÓÖŹ£¬ŹŌøł¾ŻŹµŃé»Ų“š£ŗ
£Ø1£©×¼Č·³ĘČ”ÉÕ¼īѳʷ5.0g£¬½«ŃłĘ·Åä³É250mLµÄ“ż²āŅŗ”£
£Ø2£©Č”10.00mL“ż²āŅŗ£¬ÓĆ ĮæČ”×¢Čė׶ŠĪĘæÖŠ”£(ĢīŅĒĘ÷)
£Ø3£©ÓĆ0.2000mol/L±ź×¼ŃĪĖįČÜŅŗµĪ¶Ø“ż²āÉÕ¼īČÜŅŗ£¬µĪ¶ØŹ±×óŹÖŠż×ŖĖįŹ½µĪ¶Ø¹ÜµÄ²£Į§»īČū£¬ÓŅŹÖ²»Ķ£µŲŅ”¶Æ׶ŠĪĘ棬Į½ŃŪ×¢ŹÓ £¬Ö±µ½µĪ¶ØÖÕµć”£
£Ø4£©øł¾ŻĻĀĮŠ²ā¶ØŹż¾Ż£¬·ÖĪöµĆµ½ŗĻĄķŹż¾Ż£¬¼ĘĖć“ż²āÉÕ¼īČÜŅŗµÄÅØ¶Č£ŗ ”£
| µĪ¶Ø“ĪŹż | “ż²āŅŗĢå»ż/mL | ±ź×¼ŃĪĖįĢå»ż/mL | |
| µĪ¶ØĒ°¶ĮŹż£ØmL£© | µĪ¶Øŗó¶ĮŹż£ØmL£© | ||
| µŚŅ»“Ī | 10.00 | 0.50 | 20.40 |
| µŚ¶ž“Ī | 10.00 | 4.00 | 24.10 |
| µŚČż“Ī | 10.00 | 4.20 | 25.70 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com