
������(AlN)��һ���������ǽ������ϡ�Ϊ�˷���ijAlN��Ʒ����Ʒ�е����ʲ���NaOH��Һ��Ӧ���� AlN�ĺ�����ijʵ��С���������������ʵ�鷽������֪��AlN+NaOH+H2O��NaAlO2+NH3��
������1��ȡһ��������Ʒ��������װ�òⶨ��Ʒ��AlN�Ĵ���(�г�װ������ȥ)��
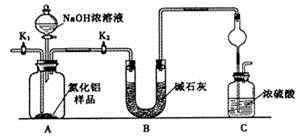
��1����ͼװ���У�U�ι�B����װ����Ϊ________��C�����θ���ܵ�������_______________________��
��2���ر�K1��K2���ٴ�Һ©������������NaOHŨ��Һ�������ٲ������塣��K1��ͨ�뵪��һ��ʱ�䣬�ⶨCװ�÷�Ӧǰ��������仯��ͨ�뵪����Ŀ����_______________________________________��
��3����������װ�û�����____________ȱ�ݣ����²ⶨ���ƫ�ߡ�
������2�������²���ⶨ��Ʒ��AlN�Ĵ��ȣ�
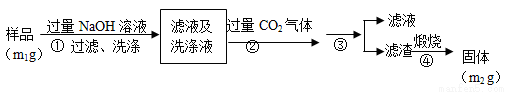
��4����������ɳ��������ӷ���ʽΪ___________________��
��5������۵IJ������õ�����Ҫ����������_________��AlN�Ĵ�����__________����m1��m2��ʾ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ��������2����ѧ�Ծ��������棩 ���ͣ�ʵ����
��֪���������Ҫ�ɷ���FeS2����Ԫ�س�+2�ۣ���Ԫ�سʡ�1�ۣ��������Ƿ�����������FeS2���������ַ������������IJ�������ͼ���£�
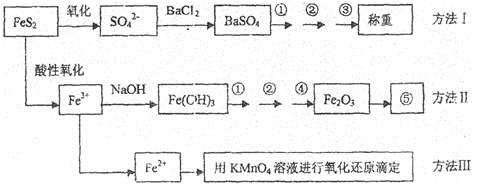
��ͬ���������⣺
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���ǣ���____________����___________����________��
�����ܡ����õ�����Ҫ�����ǣ���_________����__________(ÿ����1��2������)��
(2)�ж���Һ��SO42-���Ӽ�������ȫ�ķ�����______________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�������Ҫȷ����KMnO4����Һ���������ص��������Ƶ���ҺŨ��ƫ�����
A���������� | B������ʱ���� |
C������ʱ���ʺ�����λ�÷��ˣ���Ҫ���룩 | D������ƿ�ô�װҺ�� |
(4)ijͬѧ���÷����������ʯ�е�Fe���������ֲⶨ�������ƫ�ߣ���������Ŀ���ԭ����______________________________________��
(5)��ȡ��ʯ����1.60g����������������Ƶ�BaSO4������Ϊ4.66g�������ʯ�е���Ԫ��ȫ��������FeS2����ÿ�ʯ��FeS2������������_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ̩���и�����һ�ָ�ϰ������⣨һģ�������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����������Ԫ��W��X��Y��Z��ԭ��������������W��Zͬ�壬Y��Z���ڣ�W��Y��Z����Ԫ��ԭ�ӵ�����������֮��Ϊ11��Xԭ�������������������ڲ��������һ�롣����������ȷ����
A. ԭ�Ӱ뾶��Y>Z
B. ������ԣ�X<Y
C. ����⻯������ȶ��ԣ�Z>W
D. YԪ�ص������ﲻ����XԪ������������Ӧˮ�����ˮ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ�����и�����ѧ�ڵڶ�������������ۻ�ѧ�Ծ��������棩 ���ͣ������
���������ķ�չ���̣�Ҳ�ǻ�ѧ���ʵ���ʶ�ͷ��ֵ����̣���������������̼����ù�ء�����ء��Ҵ������ȡ����ӡ��ı����������硣
��1����ԭ���ڻ�̬ʱ����Χ�����Ų�ʽΪ____________��
��2��CO2�ĵ���ʽΪ____________��1 mol CO2�����к��ЦҼ������ʵ���Ϊ____________��
��3��6-������ù����Ľṹ��ͼ��ʾ��
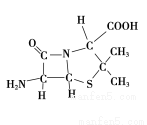
������C��N��Oԭ�Ӱ뾶�Ĵ�С��ϵΪ____________���縺�ԵĴ�С��ϵΪ____________��
�����в���sp3�ӻ���ԭ����C��____________��
��4���������NO3-�Ŀռ乹��Ϊ____________��д����NO3-��Ϊ�ȵ������һ����ǰ������Ԫ��ԭ�ӹ��ɵķǼ��Է��ӻ�ѧʽ____________��
��5���Ҵ�����Է���������������С������е��������ߣ���ԭ����____________��
��6�����Ͱ�����640��ɷ����û���Ӧ������֮һ�ľ����ṹ��ͼ��ʾ�������������Feԭ�Ӽ�ľ���Ϊa cm����þ�����ܶȼ���ʽΪ____________g/cm3����NA��ʾ�����ӵ���������
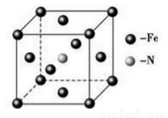
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ�����и�����ѧ�ڵڶ�������������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
���������C8H8O2����NaHCO3��Һ��Ӧ����CO2���÷��㻯��������̼ԭ���ϵ���ԭ�ӱ���ԭ��ȡ�����һ�ȴ��ﹲ�У����������칹���� ��
A. 15�� B. 16�� C. 17�� D. 18��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���������е�ʮ��У�ص���ѧ������һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£�Ksp(AgCl)=1.8��10-10, Ksp(AgI)=1.0��10-14,���������AgCl��AgI�ı�����Һ�����ϣ��������м���һ������AgNO3���壬����˵����ȷ����
A. ��AgI��Һ�м���AgNO3��c(Ag+)����Ksp(AgI)Ҳ����
B. ����Һ��ϣ�AgCl��AgI������
C. ��ȡ0.1435gAgCl�������100mLˮ����������仯����c(Cl-)Ϊ0.01mol/L
D. ��AgNO3������AgCl��AgI���ɳ���������AgClΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���������е�ʮ��У�ص���ѧ������һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ���У�Ϊʵ��ʵ��Ŀ�ı�����ӣ�������ȷ����
ʵ�� | �����Լ� | ʵ��Ŀ�� | |
�� | ��ʯ��ˮ��Ӧ | CuSO4��Һ | ��KMnO4������Һ������Ȳ�Ļ�ԭ�� |
�� | CH3CH2Br��NaOH��Һ���� | HNO3��Һ | ��AgNO3��Һ����CH3CH2Br�е�Br |
�� | ������ϡH2SO4ˮԡ���� | HNO3��Һ | ��������Һ����ˮ�����Ļ�ԭ�� |
�� | C2H5OH��ŨH2SO4������170�� | NaOH��Һ | ��KMnO4��Һ֤���÷�ӦΪ��ȥ��Ӧ |
�� | ����Һ�巴Ӧ | CCl4 | ��AgNO3��Һ֤���÷�ӦΪȡ����Ӧ |
A. �٢ڢۢܢ� B. ֻ�Тڢܢ� C. ֻ�Тڢۢ� D. ֻ�Т٢ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶�3��ѧҵˮƽ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
X��Y��Z��WΪ���ֶ���������Ԫ�أ���ԭ����������������֪X����ɻ�������������Ԫ�أ�����Yͬͬ�ڣ�Y������������������Ӳ�����3����Yԭ�ӵ�������������Wԭ��������������2����Zԭ�������ֻ��һ�����ӡ�����˵����ȷ���ǣ� ��
A. ����������Ӧˮ����ļ��ԣ�Z<W
B. ԭ�Ӱ뾶��Z>X
C. ��̬�⻯����ȶ��ԣ�X>Y
D. Y��Z���γɹ��ۻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
þȼ�ϵ����Ϊһ�ָ��ܻ�ѧ��Դ�����б������ߡ�ʹ�ð�ȫ���㡢�ɱ��͡�ȼ���������ˡ���ȾС���ص㣬ӵ�����õ�Ӧ��ǰ������ͼ��þȼ�ϵ�ص�һ��ԭ��ͼ����װ��ΪԲͲ״��������Ϊþ����ԲͲΪ�����ĵ�����ϡ������йظ�þȼ�ϵ�ص�������ȷ����
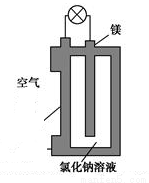
A. �õ�ص��ܷ�ӦΪ2Mg+O2=2MgO
B. ��Ӧ����O2-������������������
C. Cl-������ʧȥ��������Cl2
D. ������ӦʽΪO2+2H2O+4e-=4OH-
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com