
£Ø6·Ö£©T”ꏱ£¬ÓŠ¼×”¢ŅŅĮ½øöĆܱÕČŻĘ÷£¬¼×ČŻĘ÷µÄĢå»żĪŖ1L£¬ŅŅČŻĘ÷µÄĢå»żĪŖ2L£¬·Ö±šĻņ¼×”¢ŅŅĮ½ČŻĘ÷ÖŠ¼ÓČė6mol AŗĶ3mol
B£¬·¢Éś·“Ó¦ČēĻĀ£ŗ3A£Øg£©£«b B£Øg£© 3C£Øg£©£«2D£Øg£©£¬
3C£Øg£©£«2D£Øg£©£¬ £»4minŗó¼×ČŻĘ÷ÄŚµÄ·“Ó¦“ļµ½Ę½ŗā£¬AµÄÅضČĪŖ2.4mol/L£¬BµÄÅضČĪŖ1.8mol/L£»t minŗóŅŅČŻĘ÷ÄŚµÄ·“Ó¦“ļµ½Ę½ŗā£¬BµÄÅضČĪŖ0.8mol/L”£øł¾ŻĢāøųµÄŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£»4minŗó¼×ČŻĘ÷ÄŚµÄ·“Ó¦“ļµ½Ę½ŗā£¬AµÄÅضČĪŖ2.4mol/L£¬BµÄÅضČĪŖ1.8mol/L£»t minŗóŅŅČŻĘ÷ÄŚµÄ·“Ó¦“ļµ½Ę½ŗā£¬BµÄÅضČĪŖ0.8mol/L”£øł¾ŻĢāøųµÄŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×ČŻĘ÷ÖŠ·“Ó¦µÄĘ½¾łĖŁĀŹv£ØB£©£½_________£¬»Æѧ·½³ĢŹ½ÖŠ¼ĘĮæŹżb£½________”£
£Ø2£©ŅŅČŻĘ÷ÖŠ·“Ó¦“ļµ½Ę½ŗāĖłŠčŹ±¼ät_________4min£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©£¬ŌŅņŹĒ___________________________________________________________”£
£Ø3£©T”ꏱ£¬ŌŚĮķŅ»øöĢå»żÓėŅŅĻąĶ¬µÄ±ūČŻĘ÷ÖŠ£¬ĪŖĮĖ“ļµ½Ę½ŗāŹ±BµÄÅضČČŌČ»ĪŖ0.8mol/L£¬ĘšŹ¼Ź±£¬Ļņ±ūČŻĘ÷ÖŠ¼ÓČėC”¢DµÄĪļÖŹµÄĮæ·Ö±šĪŖ3mol”¢2mol£¬Ōņ»¹Šč¼ÓČėA”¢BµÄĪļÖŹµÄĮæ·Ö±šŹĒ___________”¢___________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
. |
| v |
| t”ę | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ?
£Ø1£©ŌŚÕā2 minÄŚ£¬¼×ČŻĘ÷ÖŠBµÄ»Æѧ·“Ó¦ĖŁĀŹĪŖ___________£¬»Æѧ·½³ĢŹ½ÖŠ»Æѧ¼ĘĮæŹżb=________”£
£Ø2£©T ”ꏱ£¬ŌŚĮķŅ»øöĢå»żÓėŅŅĻąĶ¬µÄ±ūČŻĘ÷ÖŠ£¬ĘšŹ¼Ź±¼ÓČė3 mol CŗĶ1 mol D£¬ĪŖŹ¹“ļµ½Ę½ŗāŹ±BµÄÅضČČŌČ»ĪŖ0.6 mol”¤L -1£¬Ōņ»¹Šč¼ÓČė___________mol AŗĶ____________mol B”£
£Ø3£©ČōŅŖŹ¹¼×”¢ŅŅČŻĘ÷ÖŠBµÄĘ½ŗāÅضČĻąµČ£¬æÉŅŌ²ÉČ”µÄ“ėŹ©ŹĒ____________£ØĢīŠņŗÅ£©”£
A.±£³ÖĪĀ¶Č²»±ä£¬Ōö“ó¼×ČŻĘ÷µÄĢå»żÖĮ2 L?
B.±£³ÖČŻĘ÷Ģå»ż²»±ä£¬Ź¹¼×ČŻĘ÷ÉżøßĪĀ¶Č?
C.±£³ÖČŻĘ÷Ģå»żŗĶĪĀ¶Č¶¼²»±ä£¬Ļņ¼×ČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄAĘųĢå?
D.±£³ÖČŻĘ÷Ģå»żŗĶĪĀ¶Č¶¼²»±ä£¬Ļņ¼×ČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄBĘųĢå
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźÕć½Ź”ĪĀÖŻ¶žÖŠø߶žµŚ¶žŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
£Ø6·Ö£©T”ꏱ£¬ÓŠ¼×”¢ŅŅĮ½øöĆܱÕČŻĘ÷£¬¼×ČŻĘ÷µÄĢå»żĪŖ1L£¬ŅŅČŻĘ÷µÄĢå»żĪŖ2L£¬·Ö±šĻņ¼×”¢ŅŅĮ½ČŻĘ÷ÖŠ¼ÓČė6mol AŗĶ3mol B£¬·¢Éś·“Ó¦ČēĻĀ£ŗ3A£Øg£©£«b B£Øg£© 3C£Øg£©£«2D£Øg£©£¬
3C£Øg£©£«2D£Øg£©£¬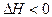 £»4minŗó¼×ČŻĘ÷ÄŚµÄ·“Ó¦“ļµ½Ę½ŗā£¬AµÄÅضČĪŖ2.4mol/L£¬BµÄÅضČĪŖ1.8mol/L£»t minŗóŅŅČŻĘ÷ÄŚµÄ·“Ó¦“ļµ½Ę½ŗā£¬BµÄÅضČĪŖ0.8mol/L”£øł¾ŻĢāøųµÄŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£»4minŗó¼×ČŻĘ÷ÄŚµÄ·“Ó¦“ļµ½Ę½ŗā£¬AµÄÅضČĪŖ2.4mol/L£¬BµÄÅضČĪŖ1.8mol/L£»t minŗóŅŅČŻĘ÷ÄŚµÄ·“Ó¦“ļµ½Ę½ŗā£¬BµÄÅضČĪŖ0.8mol/L”£øł¾ŻĢāøųµÄŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×ČŻĘ÷ÖŠ·“Ó¦µÄĘ½¾łĖŁĀŹv£ØB£©£½_________£¬»Æѧ·½³ĢŹ½ÖŠ¼ĘĮæŹżb£½________”£
£Ø2£©ŅŅČŻĘ÷ÖŠ·“Ó¦“ļµ½Ę½ŗāĖłŠčŹ±¼ät_________4min£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©£¬ŌŅņŹĒ___________________________________________________________”£
£Ø3£©T”ꏱ£¬ŌŚĮķŅ»øöĢå»żÓėŅŅĻąĶ¬µÄ±ūČŻĘ÷ÖŠ£¬ĪŖĮĖ“ļµ½Ę½ŗāŹ±BµÄÅضČČŌČ»ĪŖ0.8mol/L£¬ĘšŹ¼Ź±£¬Ļņ±ūČŻĘ÷ÖŠ¼ÓČėC”¢DµÄĪļÖŹµÄĮæ·Ö±šĪŖ3mol”¢2mol£¬Ōņ»¹Šč¼ÓČėA”¢BµÄĪļÖŹµÄĮæ·Ö±šŹĒ___________”¢___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģŠĀ½®Å©Ęߏ¦øßÖŠø߶žÉĻѧʌµŚŅ»“Ī½×¶Īæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
T”ꏱ£¬ÓŠ¼×”¢ŅŅĮ½øöĆܱÕČŻĘ÷£¬¼×ČŻĘ÷µÄĢå»żĪŖ1 L£¬ŅŅČŻĘ÷µÄĢå»żĪŖ2 L£¬·Ö±šĻņ¼×”¢ŅŅĮ½ČŻĘ÷ÖŠ¼ÓČė6 mol AŗĶ3 mol B£¬·¢Éś·“Ó¦ČēĻĀ£ŗ3A(g)£«bB(g) 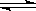 3C(g)£«2D(g)””¦¤H<0; 4 minŹ±¼×ČŻĘ÷ÄŚµÄ·“Ó¦Ē”ŗĆ“ļµ½Ę½ŗā£¬AµÄÅضČĪŖ2.4 mol/L£¬BµÄÅضČĪŖ1.8 mol/L; t minŹ±ŅŅČŻĘ÷ÄŚµÄ·“Ó¦“ļĘ½ŗā£¬BµÄÅضČĪŖ0.8 mol/L.øł¾ŻĢāøųŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
3C(g)£«2D(g)””¦¤H<0; 4 minŹ±¼×ČŻĘ÷ÄŚµÄ·“Ó¦Ē”ŗĆ“ļµ½Ę½ŗā£¬AµÄÅضČĪŖ2.4 mol/L£¬BµÄÅضČĪŖ1.8 mol/L; t minŹ±ŅŅČŻĘ÷ÄŚµÄ·“Ó¦“ļĘ½ŗā£¬BµÄÅضČĪŖ0.8 mol/L.øł¾ŻĢāøųŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¼×ČŻĘ÷ÖŠ·“Ó¦µÄĘ½¾łĖŁĀŹv(B)£½________£¬»Æѧ·½³ĢŹ½ÖŠ¼ĘĮæŹżb£½________.
(2)ŅŅČŻĘ÷ÖŠ·“Ó¦“ļµ½Ę½ŗāŹ±ĖłŠčŹ±¼ät________4 min(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)£¬ŌŅņŹĒ______________________________£®
(3)T”ꏱ£¬ŌŚĮķŅ»øöĢå»żÓėŅŅĻąĶ¬µÄ±ūČŻĘ÷ÖŠ£¬ĪŖĮĖ“ļµ½Ę½ŗāŹ±BµÄÅضČČŌČ»ĪŖ0.8 mol/L£¬ĘšŹ¼Ź±£¬Ļņ±ūČŻĘ÷ÖŠ¼ÓČėC”¢DµÄĪļÖŹµÄĮæ·Ö±šĪŖ3 mol”¢2 mol£¬Ōņ»¹Šč¼ÓČėA”¢BµÄĪļÖŹµÄĮæ·Ö±šŹĒ________”¢________.
(4)ČōŅŖŹ¹¼×”¢ŅŅČŻĘ÷ÖŠBµÄĘ½ŗāÅضČĻąµČ£¬æÉŅŌ²ÉČ”µÄ“ėŹ©ŹĒ________£®
A£®±£³ÖĪĀ¶Č²»±ä£¬Ōö“ó¼×ČŻĘ÷µÄĢå»żÖĮ2 L
B£®±£³ÖČŻĘ÷Ģå»ż²»±ä£¬Ź¹¼×ČŻĘ÷ÉżøßĪĀ¶Č
C£®±£³ÖČŻĘ÷Ń¹ĒæŗĶĪĀ¶Č¶¼²»±ä£¬Ļņ¼×ÖŠ¼ÓČėŅ»¶ØĮæµÄAĘųĢå
D£®±£³ÖČŻĘ÷Ń¹ĒæŗĶĪĀ¶Č¶¼²»±ä£¬Ļņ¼×ÖŠ¼ÓČėŅ»¶ØĮæµÄBĘųĢå
(5)Š“³öĘ½ŗā³£Źż±ķ“ļŹ½K£½________£¬²¢¼ĘĖćŌŚT”ꏱµÄ»ÆŃ§Ę½ŗā³£ŹżK£½________.
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com