

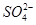 µÄæռ乹ŠĶĪŖ_________£¬H2OÖŠOŌ×ÓµÄŌӻƷ½Ź½ĪŖ____________”£
µÄæռ乹ŠĶĪŖ_________£¬H2OÖŠOŌ×ÓµÄŌӻƷ½Ź½ĪŖ____________”£ ÅäĄė×Ó”£ŅŃÖŖ
ÅäĄė×Ó”£ŅŃÖŖ µÄæռ乹ŠĶ¶¼ŹĒČż½Ē׶ŠĪ£¬µ«NF3²»Ņ×ÓėCu2+ŠĪ³ÉÅäĄė×Ó£¬ĘäŌŅņŹĒ____________________________”£
µÄæռ乹ŠĶ¶¼ŹĒČż½Ē׶ŠĪ£¬µ«NF3²»Ņ×ÓėCu2+ŠĪ³ÉÅäĄė×Ó£¬ĘäŌŅņŹĒ____________________________”£ NŠĪ³ÉµÄ¾§Ģå½į¹¹ČēĶ¼ĖłŹ¾£¬N3-µÄÅäĪ»ŹżŹĒ________”£Éč¾§°ū±ß³¤ĪŖa cm£¬ĆܶČĪŖb g/cm3£¬Ōņ°¢·ü¼ÓµĀĀŽ³£ŹżæɱķŹ¾ĪŖ___________(ÓĆŗ¬a”¢bµÄŹ½×Ó±ķŹ¾)”£
NŠĪ³ÉµÄ¾§Ģå½į¹¹ČēĶ¼ĖłŹ¾£¬N3-µÄÅäĪ»ŹżŹĒ________”£Éč¾§°ū±ß³¤ĪŖa cm£¬ĆܶČĪŖb g/cm3£¬Ōņ°¢·ü¼ÓµĀĀŽ³£ŹżæɱķŹ¾ĪŖ___________(ÓĆŗ¬a”¢bµÄŹ½×Ó±ķŹ¾)”£
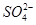 µÄæռ乹ŠĶÓ¦ĪŖÕżĖÄĆęĢåŠĶ£¬H2OÖŠOŌ×ÓµÄŌӻƷ½Ź½ĪŖsp3£»£Ø3£©ŅņĪŖFµÄµēøŗŠŌ±ČN“ó£¬N-F³É¼üµē×Ó¶ŌĘ«ĻņFŌ×Ó£¬Ź¹µĆµŖŌ×ÓÉĻµÄ¹Ā¶Ōµē×ÓÄŃÓėCu2+ŠĪ³ÉÅäĄė×Ó£»£Ø4£©øł¾Ż¾§°ū½į¹¹æÉÖŖ¶„µćĪ»ÖĆĪŖN3-£¬ĄćÉĻĪŖCu+£¬¹ŹN3-µÄÅäĪ»ŹżŹĒ6£»1øö¾§°ūÖŠŗ¬ÓŠ3øöCu+ŗĶ1øöN3”Ŗ£¬¹Ź1øö¾§°ūµÄÖŹĮæĪŖ206/NA g£¬Ģå»żĪŖa3 cm3£¬ĖłŅŌĆܶČbg/cm3=206/NA g”Āa3 cm3£¬¹ŹNA=206/a3b”£
µÄæռ乹ŠĶÓ¦ĪŖÕżĖÄĆęĢåŠĶ£¬H2OÖŠOŌ×ÓµÄŌӻƷ½Ź½ĪŖsp3£»£Ø3£©ŅņĪŖFµÄµēøŗŠŌ±ČN“ó£¬N-F³É¼üµē×Ó¶ŌĘ«ĻņFŌ×Ó£¬Ź¹µĆµŖŌ×ÓÉĻµÄ¹Ā¶Ōµē×ÓÄŃÓėCu2+ŠĪ³ÉÅäĄė×Ó£»£Ø4£©øł¾Ż¾§°ū½į¹¹æÉÖŖ¶„µćĪ»ÖĆĪŖN3-£¬ĄćÉĻĪŖCu+£¬¹ŹN3-µÄÅäĪ»ŹżŹĒ6£»1øö¾§°ūÖŠŗ¬ÓŠ3øöCu+ŗĶ1øöN3”Ŗ£¬¹Ź1øö¾§°ūµÄÖŹĮæĪŖ206/NA g£¬Ģå»żĪŖa3 cm3£¬ĖłŅŌĆܶČbg/cm3=206/NA g”Āa3 cm3£¬¹ŹNA=206/a3b”£

 ÓŵČÉśĢāæāĻµĮŠ“š°ø
ÓŵČÉśĢāæāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| | | m | n |
| x | y | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®CH4ÓėSiH4µÄĪČ¶ØŠŌ£ŗCH4£¾SiH4 |
| B£®HClOÓėH2SO4µÄĖįŠŌ£ŗHClO £¾H2SO4 |
| C£®1molHCl£Øg£©µÄÄÜĮæ±Č1molH2(g)ŗĶ1molCl2(g)µÄÄÜĮæŗĶøß |
| D£®ClÓėSµÄŌ×Ó°ė¾¶£ŗCl £¾S |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
| ”” | ¢Ł | ¢Ś | ¢Ū | ¢Ü | ¢Ż | ¢Ž | ¢ß | ¢ą | ¢į | ¢ā |
| Ō×Ó°ė¾¶/10 -10m | 0.37 | 1.86 | 0.74 | 1.43 | 0.77 | 1.10 | 0.99 | 1.52 | 0.75 | 0.71 |
| ×īøß¼ŪĢ¬ | +1 | +1 | ”” | +3 | +4 | +5 | +7 | +1 | +5 | ”” |
| ×īµĶ¼ŪĢ¬ | -1 | ”” | -2 | ”” | -4 | -3 | -1 | ”” | -3 | -1 |
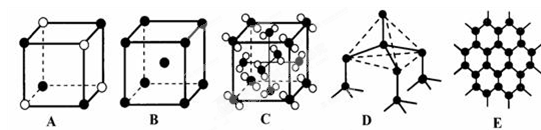
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ęä×īøß»ÆŗĻ¼ŪĪŖ+3 | B£®æÉŅŌŠĪ³É»ÆѧŹ½ĪŖKXO3µÄŃĪ |
| C£®ĘäĒā»ÆĪļæÉŅŌÓĆĄ“×öÅēČŖŹµŃé | D£®Ęä×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļŹĒĒæĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®O”¢Na”¢SµÄŌ×Ó°ė¾¶ŅĄ“ĪŌö“ó | B£®LiOH”¢KOH”¢CsOHµÄ¼īŠŌŅĄ“ĪŌöĒæ |
| C£®HF”¢NH3”¢SiH4µÄĪČ¶ØŠŌŅĄ“ĪŌöĒæ | D£®HCl”¢HBr”¢HIµÄ»¹ŌŠŌŅĄ“Ī¼õČõ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com