
çÓÑóöÜáÁøŤ˜ÆÅCr(OH)3ÀÂAl2O3ÀÂZnOÀÂCuOÀÂNiOçàöÿøò,¿ÊØçèüë´¿»À¯øÅöôݤèíÀˆáóî¾£₤ñ´ÀÝ£ÄòíNa2Cr2O7çàöÿøòÀÈ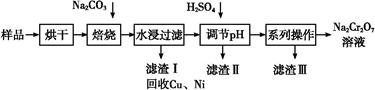
Øîøˆ:åÖNa2CrO4àÉؤøŤ˜ÆÅèìê¢NaAlO2ÀÂNa2ZnO2çàöÿøò
(1)ùÛ§±¤µçáàÉؤ°òÀÀÀÀÀÀÀÀÅå(äŸÀ¯ùÃÀÝÀÂÀ¯¥ŸÀÝ£·À¯øÅÀÝ)ÀÈ
(2)ëõ°èî¾£₤ݤèí¿»°äøÅ躰èNa2CrO4çá£₤îÏñ§°äò§ÀÈ
ÀÀÀÀÀÀÀÀCr(OH)3+ÀÀÀÀÀÀÀÀNa2CO3+ÀÀÀÀÀÀÀÀÀÀÀÀ ÀÀÀÀÀÀÀÀNa2CrO4+ÀÀÀÀÀÀÀÀCO2+ÀÀÀÀÀÀÀÀÀÀÀÀ
ÀÀÀÀÀÀÀÀNa2CrO4+ÀÀÀÀÀÀÀÀCO2+ÀÀÀÀÀÀÀÀÀÀÀÀ
(3)ôùå■·çáø¼Øˆ°èñøÆÅZn(OH)2ÀÂÀÀÀÀÀÀÀÀÀÈ
(4)À¯üçêÅýìæ¼ÀÝøÅöˆ:¥äŽ¥ÆàŠH2SO4,ÀÀÀÀÀÀÀÀ,âðàǧÃÏ,¿»ôùÀȥ䎥ÆàŠH2SO4á¢çáòúÀÀ ÀÈ
Øîøˆ:Âì°»àËôùå■II¤µ,àÉؤøÅÇÌåÖàÓüôñÇÆÎ:
2CrO42Àˆ+2H+ Cr2O72Àˆ+H2O
Cr2O72Àˆ+H2O
ÂÖNa2Cr2O7ÀÂNa2CrO4åÖý£ë˜öôÑàüôçáàɧãÑààÓüôÝÚ
| öôÑà àɧãÑà £₤îÏò§ | 20 ÀÌ | 60 ÀÌ | 100 ÀÌ |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
| Na2CrO4 | 84 | 115 | 126 |
 åáÑꢚ°çüçêÅÇÞ¯¡
åáÑꢚ°çüçêÅÇÞ¯¡
| áõ¥Ñ | ¡ÔøÅ¢ö°ä | áõ¥Ñ | °¾øÅ¢ö°ä |
| ¡ÔØ£ | ¡ÔØ£ûãñî¢ö°äëó¥—ÈÀ | °¾Ø£ | °¾Ø£ûãñî¢ö°äëó¥—ÈÀ |
| ¡Ôѱ | ¡Ôѱûãñî¢ö°äëó¥—ÈÀ | °¾Ñ± | °¾Ñ±ûãñî¢ö°äëó¥—ÈÀ |
| ¡Ôà» | ¡Ôà»ûãñî¢ö°äëó¥—ÈÀ | °¾à» | °¾à»ûãñî¢ö°äëó¥—ÈÀ |
¢óá¢È¤¡ÔøÅ£₤îÏ âÇåÇȤ äãÅëȤ䟢íäã
á¢ú¯òâ§Óèü60%çáûƒòúÇƤÈùÛøÅäÃàÀçáÀȤÈùÛäÃûƒçáø¼Øˆê¼°äàÓüô:
úŠ£ÄÇÞüôêÅöòäã:
(1)ÇÆâŠæÆñÇÆÎçá§úÑàù¥¢¥,åÖ¤ÈùÛøÅ¥ÆàŠò₤£ØàÕçáæ¼ÆûòúÀÀÀÀÀÀÀÀ,ÅÇ°—åÖ°êçÚ°ÄøÅñÂèºñÇÆÎçáâŠæÆñ§°äò§ÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÀÈ
(2)ò₤£ØàÕòúèºò₤£ØÆŠùÛÅö°èçá£₤¤üöÿ,ÇÆ°ðñøâ«Æû¤Èîµ£₤îÏæòåÇ,äáԃ٥ûÅÏØÌçá§úÑà,èºýºèºò₤£Øçáø¼ØˆåÙêüâÇåÇÆÖ¤ÈîµøÅçáÀÀÀÀÀÀÀÀÀÈ
(3)ýìæ¼AòúÀÀÀÀÀÀÀÀ,ýìæ¼BòúÀÀÀÀÀÀÀÀÀÈ
(4)¥ÆàŠçáæÐê¢òå¥êaòúÀÀÀÀÀÀÀÀ(䟣₤îÏò§)ÀÈ
(5)ößùÛMgCl2åÖàÜàÖæÇä˜üô,ë´çÓ¤µ£ÃýºèºMg¤ëCl2,¡ûñÇÆÎçá£₤îÏñ§°äò§öˆÀÀÀÀÀÀÀÀÀÀÀÀÀÈÇÆ¢¥ôú°è݃¤ëñüöÿîÙ£ñâ«Æûçá§úÑà,¡Ýýºöÿôàó½¢èØåÆûÆÖÀÀÀÀÀÀÀÀÀÈ
(6)¤ÈùÛäÃûƒçá¿»°ä,öˆòýûÇ؈§¨¤ÈùÛøÅçáôà£₤ûƒæˆÝðöˆúãî¾£₤ûƒ,åìæˆÝðöˆôà£₤ûƒ?
_____________________________________________
ýÕ¢ÇÇÞ¯¡¤ë§ãö—>>
¢óá¢È¤¡ÔøÅ£₤îÏ âÇåÇȤ äãÅëȤòçîÕäã
Àƒ£₤îÏÀˆîÀÅß2Ȥ£₤îÏÆŠ¥¥ò¾À¢È´15ñøÈˋ
èºä˜¿ÊØçå¯ú½ç᧴èÒȘý£§—§—òúäÍüø£ñÝÈâÚ១■؈ØâƒïîÙ£ñƒÙ¥ûâÚôܤë°ðñø¢¥ôúƒÙ¥ûçá¢è°øŽñÂí¿ÀÈüôûÌòúá°óµØçèÒ¥óçáê·ùÃÈÙêæÿÏÈÙùÛáÁêˆýºÈ˜¤ÈùÛÈÙçÙùÛÑÁÆûȘîöÈÙààÈÙçÓêˆýºà»Çµèºä˜ýºØçêÇê¼°äë¥ÀÈ
¡ªƒïèüò—ýºØçê¼°ä£ÄÇÞüôêÅöòäãȤ
È´1ÈˋÇÆåÙêüÀÂáÉåÇÀ§£ë´§úÑࢥôú¡ûóµØçÆΧ´åÖÈ´ÀÀÀÀÈˋ
ÀÀÀÀ
| AÈÛö¼ý¢è§ú½ÀÀÀÀ | BÈÛîĤÈçÄú½ÀÀÀÀ | CÈÛñÂÇÿ°úòÅÀÀÀÀ | DÈÛѨÝÝáÖô§ |
ýÕ¢ÇÇÞ¯¡¤ë§ãö—>>
¢óá¢È¤¡ÔøÅ£₤îÏ âÇåÇȤ äãÅëȤ䟢íäã
¿ÊØçèüØå£ó亢µöˆåÙêüèºýºê·ùÃø¼Øˆñøöˆà»¡—§æÑö§½ÅÅȘ¥ÇšîèíÀÂÇÔ£₤î¾£₤ÀÂö■òíÀÈúŠ£ÄÇÞüôêÅöòäãȤ
(1)šîèí£ó亢µÅö°èçáô₤ó½ÝÄÅŠƒÙ°»°ƒÀÂüÇçÆÀ¡èåÿ¤µ§½àŠ (äŸèÒÝ¡û«°ó)Șóðø¼Øˆá¢çáòú ÀÈ
(2)ÇÔ£₤î¾£₤ùªò¿ÆûçáÇÔ£₤¥êñ¯ÇËû§(V2O5)áɥƢšÑ±î¾£₤ê·î¾£₤ùìôòȘÇù¿»°äøÅýºèºêùØ£ê˜ÇÛçáøÅ¥ðäÍ(àÓë¥1)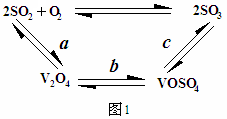
óðøÅaÀÂcѱý§ñÇÆÎçá£₤îÏñ§°äò§¢èÝÚòƒöˆÈ¤ À ÀÈ
(3)550ÀÌòÝȘSO2戣₤öˆSO3çáó§¤ã戣₤ôò(Îê)ÆŠäÍüçæÉî¿ú¢(P)çá¿ÄüçàÓë¥2ùªòƒÈ˜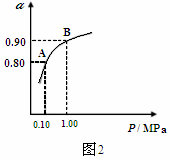
å·È¤§¨2.0mol SO2¤ë1.0mol O2øûÆÖ5LûÉÝíàïó¼øÅȘñÇÆÎÇÿó§¤ã¤µÈ˜äÍüçæÉî¿ú¢öˆ0.10M PaȘAÆŠBÝÚòƒý£ë˜î¿ú¢üôçáSO2戣₤ôòȘ봰ÈúÕ¢—üô¿ÊØçèºýºøÅýèÆû°Èî¿çáåÙØ·òúȤ ÀÈ
(4)öˆîÙ£ñâ«ÆûÇÔ£₤¥êȘ¢óîÅàùåÝæŸÅôîÅøóêùØ£øøâŠæƧ£££ñ´£Äòíñ¯çáÅô¿ÊØíȘ£ÄòíôòÇÿ91.7%ØåèüÀÈØîøˆñüñ¯ÇÔ£₤¥êøŤ˜ÆÅV2O5ÀÂVOSO4¥¯ý£àÉÅåýÅå■ȘýÕåáæòêüøˆÈ¤VOSO4¢èàÉÆÖùÛȘV2O5áîàÉÆÖùÛȘNH4VO3áîàÉÆÖùÛÀÈ¡û¿ÊØíçáê¼°äàÓë¥àÓüôȤ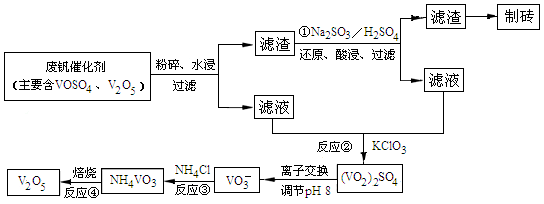
å·ñÇÆÎÂìÂÖÂÜÂÉøÅò¶ÆÖî¾£₤£¿åÙñÇÆÎçáòú (äŸò»æøÅ·¤é)ȘñÇÆÎÂìçáâŠæÆñ§°äò§öˆ Ș¡û¿ÊØíøÅñÇÆÎÂÜçá°êçÚôò(Æø°ó°êñ¯ôò)òú£Äòíñ¯çá¿Ä¥■øÛأȘ°êñ¯ôòçá¡Ôçë°»òÉàÉؤpHƯüšëãȘ£¿ÅÒ؈¢Äøóôà£₤ÿÏüçò»(NH4Cl¥ÆàŠøòê¢ÆŠêüؤøÅV2O5çáøòê¢Ýà)¤ëöôÑàÀÈ¡ªƒïüôë¥ò姴ØÕ¢Äøóôà£₤ÿÏüçò»¤ëöôÑàȤ À ÀÈ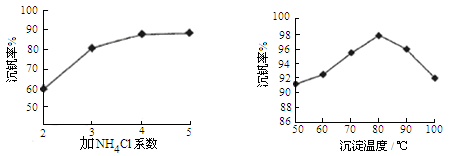
ýÕ¢ÇÇÞ¯¡¤ë§ãö—>>
¢óá¢È¤¡ÔøÅ£₤îÏ âÇåÇȤ äãÅëȤ䟢íäã
Ý«ùÃçá§Ã¿¿öˆCH3ÀˆCH2ÀˆCOOH,Ý«ùÃîöòú¯ýà¨ÆÅÅÏçáñâû¿ÀÂñâ¡₤¥ê,Ø£øøØ奟ò§ä¥ùÃÅ¢öˆåÙêüçáèºýº¿ÊØíê¼°äàÓüô: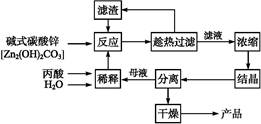
| Å·¤é | n(Ý«ùÃ)Àû n(¥Ÿò§ä¥ùÃÅ¢) | ñÇÆÎöôÑà/ÀÌ | Ý«ùÃÅ¢ýºôò/% |
| 1 | 1Àû0.25 | 60 | 67.2 |
| 2 | 1Àû0.25 | 80 | 83.5 |
| 3 | 1Àû0.25 | 100 | 81.4 |
| 4 | 1Àû0.31 | 60 | 89.2 |
| 5 | 1Àû0.31 | 80 | 90.1 |
| 6 | 1Àû0.31 | 100 | 88.8 |
 ,ñÇÆÎöôÑàÀÀÀÀÀÀÀÀÀÌÀÈ
,ñÇÆÎöôÑàÀÀÀÀÀÀÀÀÀÌÀÈ ýÕ¢ÇÇÞ¯¡¤ë§ãö—>>
¢óá¢È¤¡ÔøÅ£₤îÏ âÇåÇȤ äãÅëȤ䟢íäã
§Þò¶ŸîÈ´TiÈˋØ·óðÆýÑàǵÀÂàÜçСÔÀ°ÈöôòÝáëùÃ¥Ÿ¡₤òÇѽݣ¿Ðñ¤Æû漡ÔÅô¢ó¥¥ýáêüȘݣƱöˆÀ¯öÇâǧÞò¶ÀÝÀÈØåŸî亢µÈ´ø¼Øˆ°èñøFeTiO3ȘŸîùÃîúäºÈˋöˆø¼ØˆåÙêüØÝêѧÞò¶Ÿîë˜òÝ£þçû¡Ýýºóñ¥æçá¿ÊØçèºýºê¼°äàÓüôȘ£ÄÇÞüôêÅöòäãȤ
È´1ÈˋŸî亢µ¤ëé´ê·ùÃñÇÆÎçáýºöÿøÛØ£òúTiOSO4ȘñÇÆÎøÅößó½äÍ躰èÀÈ¡Ýýºóñ¥æùæ°óÀ¯ôäñ₤ÀÝóð£₤îÏò§òú________________ÀÈ
È´2Èˋèüò—èºýºê¼°äøÅ¥ÆàŠFeÅ¥çáá¢çáòú È´ÆûâŠæÆñ§°äò§ÝÚòƒÈˋȘ¥šîÕ¡Ýýºóñ¥æòúñþÝðøòçáòçîÕñ§ñ´òú ÀÈ
È´3Èˋèüò—èºýºê¼°äøÅùªçûç§çá§Þò¶ŸîøÅ£šÆÅèìê¢åÆøòȘ¢è¥ÆàŠ àɧ㤵°»àËÀÈ
È´4ÈˋàÉؤÂþøŤ˜ÆÅFe2+ÀÂTiO2+¤ëèìê¢Mg2+çàî¶âŠæÆÀÈ°ÈöôüôȘóðÑåÆÎúãî¾£₤öÿçáKspàÓüôÝÚùªòƒÀÈ
| úãî¾£₤öÿ | FeÈ´OH)2 | TiO(OH)2 | Mg(OH)2 |
| Ksp | 8.0Àê10-16 | 1.0Àê10-29 | 1.8Àê10-11 |

ýÕ¢ÇÇÞ¯¡¤ë§ãö—>>
¢óá¢È¤¡ÔøÅ£₤îÏ âÇåÇȤ äãÅëȤ䟢íäã
Ý«ùÃçá§Ã¿¿öˆCH3ÀˆCH2ÀˆCOOH,Ý«ùÃîöòú¯ýà¨ÆÅÅÏçáñâû¿ÀÂñâ¡₤¥ê,Ø£øøØ奟ò§ä¥ùÃÅ¢öˆåÙêüçáèºýº¿ÊØíê¼°äàÓüô:
| Å·¤é | n(Ý«ùÃ)Àû n(¥Ÿò§ä¥ùÃÅ¢) | ñÇÆÎöôÑà/ÀÌ | Ý«ùÃÅ¢ýºôò/% |
| 1 | 1Àû0.25 | 60 | 67.2 |
| 2 | 1Àû0.25 | 80 | 83.5 |
| 3 | 1Àû0.25 | 100 | 81.4 |
| 4 | 1Àû0.31 | 60 | 89.2 |
| 5 | 1Àû0.31 | 80 | 90.1 |
| 6 | 1Àû0.31 | 100 | 88.8 |
 ,ñÇÆÎöôÑàÀÀÀÀÀÀÀÀÀÌÀÈ
,ñÇÆÎöôÑàÀÀÀÀÀÀÀÀÀÌÀÈ ýÕ¢ÇÇÞ¯¡¤ë§ãö—>>
¢óá¢È¤¡ÔøÅ£₤îÏ âÇåÇȤ äãÅëȤ䟢íäã
ѱ₤Ÿî¿Ðñ¤ÆÎÆûÆÖ¡¼âÁ§Ã¿¿ÝÚûÌë¢êüÀÂø§íéë¢ýÐçàȘѱ₤Ÿî£¿¢èæ¼öˆøóÝ¡ŸîçËøòçáåÙêüÀÈ
ÂþÈÛѱ₤Ÿî¢èÆèØåüôê§øøñ§ñ´øóݡȤ
ñ§ñ´1ȤTiCl4ùÛ§ã躰èTiO2ÀÊxH2OȘ¿»ôùÀÂùÛüÇ°»àËóðøÅçáClÈÙȘåì¤Ì¡èÀÂݤèí°»àËùÛñøçûç§ñÜäÍTiO2ȘÇùñ§ñ´øóÝ¡çûç§çáòúáèûæѱ₤ŸîÀÈ
È´1ÈˋÂì TiCl4ùÛ§ã躰èTiO2ÀÊx H2Oçá£₤îÏñ§°äò§öˆ_______________________________È£
ÂÖ ¥šîÕTiO2ÀÊx H2OøÅClÈÙòúñþÝ£°»ƒ£çáñ§ñ´òú______________________________ÀÈ
ñ§ñ´2Ȥ¢èÆû¤˜ÆÅFe2O3çáŸî亢µÈ´ø¼Øˆ°èñøöˆFeTiO3ȘóðøÅTiåˆùÄ£₤¤ü¥Üöˆ+4¥ÜÈˋøóàÀȘóðø¼Øˆê¼°äàÓüôȤ
È´2ÈˋFe2O3ÆŠH2SO4ñÇÆÎçáâŠæÆñ§°äò§òú ÀÈ
È´3Èˋ¥æàÉؤøÅ°»¤˜TiO2+øÛë㣿¤˜ÆÅçá§Þò¶î¶âŠæÆÆÅ ÀÈ
È´4Èˋ¥ÆFeçáæ¼Æûòú ÀÈ
·.ѱ₤Ÿî¢èÆûÆÖøóàÀŸîçËøò
È´5ÈˋTiO2øóàÀçËøòTiȘèÌ¥¯ç§çáý§øÒàÓüôȤ
ñÇÆÎÂÖçáñ§°äò§òú Ș¡ûñÇÆÎÅÒ؈åÖAró½ñíøŧ½ÅÅȘúŠ§ãòëåÙطȤ_____________ÀÈ
ýÕ¢ÇÇÞ¯¡¤ë§ãö—>>
¢óá¢È¤¡ÔøÅ£₤îÏ âÇåÇȤ äãÅëȤçËîÀäã
üôêÅöÿøòçáøóݡȘñ«¤ü¿ÊØçèºýºòç¥òçáòú( )
| AÈÛ§¨ôàó½ë´àŠ°öúÍò₤£ØùÛøÅøóó₤¯æñÜ |
| BÈÛÆûâŠæƧ£££áÊñ´çÓ§ãÝˤëò°îöùÛøóÝ¡èí¥ŸÀÂúãó½¤ëôàó½ |
| CÈÛ§¨úãó½¤ëôà󽣚¤ü¤µçÐà¥È˜ýºöÿÆûùÛö■òíøóÝ¡îöùà |
| DÈÛ§¨SO2¤ëO2çᣚ¤üó½¥Æ¡ÔµÈ˜ë´¿»§ÆÇËòØȘøóÝ¡SO3 |
ýÕ¢ÇÇÞ¯¡¤ë§ãö—>>
¯ìÑàøôÅé - êñü¯ýÃêÅÝÚ - òåäãêÅÝÚ
¤±ÝÝòÀ£Ëêˆë½öËñ´¤ëý£ê¥ÅéüƒìÝ´ó§ä´ | ë½èüÆŤÎÅéüƒìÝ´æ´ú½ | çÓÅéíˋóÙƒìÝ´æ´ú½ | èÌâºòñÅÕößø¼ØÍÆŤÎÅéüƒìÝ´æ´ú½ | èÌóµúøà´ƒìÝ´æ´ú½
öËñ´¤ëý£ê¥ÅéüƒìÝ´çÓ£¯È¤027-86699610 ƒìÝ´ÆòüðȤ58377363@163.com