
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
(1)��ʳ�γ���������Ca2����Mg2����SO42�����������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba(NO3)2��Һ
(1)������ȥ��Һ���е�Ca2����Mg2����SO42����ѡ��A���������Լ������μ�˳������Ϊ (ֻ�ѧʽ)��������Һ��SO42��������ȫ�IJ���Ϊ ��
�ڼ������Ŀ����(�����ӷ���ʽ��ʾ) ��
�����������У��ס����������̾�Ҫ�õ��������������������÷ֱ��Ǽ� ���� ��
(2)��ҵ���ö��Ե缫��ⱥ��ʳ��ˮ����ȡ���ᣬд����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ ������ֽ������ʳ��ˮ��������ķ����� ��
��1����BaCl2��NaOH ��Na2CO3��NaOH��BaCl2��Na2CO3
�Դ�Ƭ�̣�������������ϣ��ϲ������Һ�����ϲ���Һ�м����μ��Ȼ�����Һ���������ɣ��������ȫ��
��OH����H��=H2O CO32����2H��=H2O��CO2��
�۽�������
��2��2NaCl��2H2O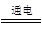 2NaOH��H2����Cl2��
2NaOH��H2����Cl2��
��ʪ�����ɫʯ����ֽ���������������У���ֽ�ȱ�죬����ɫ��֤������������(����ʪ���KI-������ֽ���������������У���ֽ����ɫ)
���������������1������Ca2+��Na2CO3��Һ������Mg2+��NaOH��Һ������SO42-��BaCl2��Һ����Ҫע������Լ���������ҹ������Լ��������������ȥ�������Լ��μӵ�˳����BaCl2��NaOH ��Na2CO3��NaOH�� BaCl2��Na2CO3������SO42-������ȫ�IJ�������Ϊ����Һ���ã����ϲ���Һ�м����μ��Ȼ�����Һ���������ɣ�˵��SO42-��ȫ��������Һ�м�����������dz�ȥ������CO32-��OH-���ܽ�ʱ�ò��������裬�ӿ��ܽ��ٶȡ�����ʱ�ò�����������
��2����ⱥ��ʳ��ˮ�����������ơ�������������������������������ʪ�����-KI��ֽ���顣
���㣺��ѧʵ�� ��� Ԫ�ؼ��仯����
����������ʱҪע���Լ������˳���������Լ���������������Լ��������������ȥ��


 �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ը����ʱ1 kg��������0.5 kgˮ��4 g������10 gС�մ�����ʳ�εȸ��ϣ�����ը����Ʒ�����IJ���һ��Ϊ80%��ͨ������˵����ÿ��ʳ��100 g��������������������__________________��?
��2�����о��ҹ��������ճ�����������������ʳƷ���Ӽ��⣩�����ֿ���;����______________________________________________________________________?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0115 �¿��� ���ͣ������
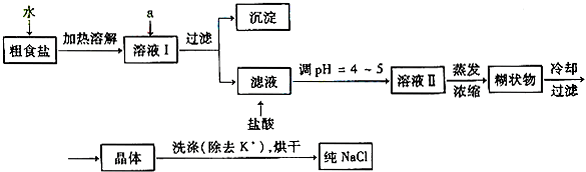
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com