���������ڹ�ũҵ�����о�����Ҫ����;��ijС����ݹ�ҵ����ԭ����������������������Ҫ�������£�
��1����N
2��H
2Ϊԭ�Ϻϳɰ�������ӦN
2��g��+3H
2��g��?2NH
3��g����H��0
��һ���¶��£����ܱ������г���1mol N
2��3mol H
2������Ӧ���������ݻ��㶨���ﵽƽ��״̬ʱ������������ʵ�����ԭ����
����N
2��ת���ʦ�=
������ʱ�ų�����Ϊa KJ�������Ȼ�ѧ����ʽΪ
��
�ڰ�������ˮ��Ϊ��ˮ����֪NH
3?H
2O�ĵ���ƽ�ⳣ��ΪKb������0.1mol/L��NH
3?H
2O��Һ��c��OH
-��=
mol/L����ƽ��ʱNH
3?H
2O��Ũ��ԼΪ0.1mol/L���ú���Kb�Ĵ���ʽ��ʾ����
�ۺϳɰ���ԭ����H
2������CH
4���ۺ����ã�CH
4+H
2O�TCO+3H
2����CH
4��Ƴ�ȼ�ϵ�أ��������ʸ��ߣ�װ��ʾ����ͼһ��A��BΪ�����ʯī����������ͨ����飬�ڱ�״���£����ļ������VL��44.8L��V��89.6Lʱ�������缫��ӦΪ
��
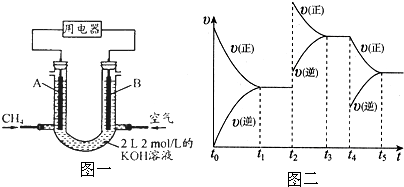
��2������������Ϊ��Ҫԭ���Ƚ��а��Ĵ�������Ȼ���Ƶ����ᣮ
������NO���ݻ��㶨���ܱ������н��з�Ӧ��2NO��g��+O
2��g��?2NO
2��g����H��0
�÷�Ӧ�ķ�Ӧ���ʣ�v����ʱ�䣨t���仯�Ĺ�ϵ��ͼ����ʾ����t
2��t
4ʱ��ֻ�ı�һ������������˵����ȷ���ǣ���ѡ����ţ�
��
a����t
1��t
2ʱ��������������������ܶȱ��ֲ����жϷ�Ӧ�Ѵﵽƽ��״̬
b����t
2ʱ����ȡ�Ĵ�ʩ�����������¶�
c����t
3��t
4ʱ����t
1��t
2ʱ��ƽ�ⳣ��K�϶���ͬ
d����t
5ʱ��������NO
2��������������������е����ֵ
��ʵ���ϣ����ɵ�NO
2��ۺ�����N
2O
4�������һ�ܱ������У�17�桢1.01��10
5Pa�����£�2NO
2��g��?N
2O
4��g����H��0��ƽ�ⳣ��K=13.3�����ı�������ϵ��ij���������ﵽ�µ�ƽ���û��������c��NO
2��=0.04mol/L��c��N
2O
4��=0.007mol/L����ı��������
��
�����᳧�������·�������β����Na
2CO
3��Һ����NO
2����CO
2����ÿ9.2g NO
2��Na
2CO
3��Һ��Ӧʱת�Ƶ�����Ϊ0.1mol����Ӧ�����ӷ���ʽ��
��



 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�

 ���÷�Ӧ��Cu2Cl2+C2H2+2NH3��Cu2C2����Ȳ��ͭ����ɫ��+2NH4Cl�ɼ�����Ȳ��
���÷�Ӧ��Cu2Cl2+C2H2+2NH3��Cu2C2����Ȳ��ͭ����ɫ��+2NH4Cl�ɼ�����Ȳ��



 ���С������г��漰�ơ����仯�����ͼΪ������ܵ�صĽṹʾ��ͼ���õ�صĹ����¶�Ϊ320�����ң���ط�ӦΪ2Na+xS�TNa2Sx����������������ǣ�������
���С������г��漰�ơ����仯�����ͼΪ������ܵ�صĽṹʾ��ͼ���õ�صĹ����¶�Ϊ320�����ң���ط�ӦΪ2Na+xS�TNa2Sx����������������ǣ�������