
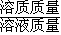 ×100%��������ͭ��Һ�������������ݴ��жϣ�
×100%��������ͭ��Һ�������������ݴ��жϣ�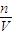 �жϣ�
�жϣ� �жϣ�
�жϣ� =6.4g���ʸ�����ͭ��Һ����������Ϊ
=6.4g���ʸ�����ͭ��Һ����������Ϊ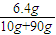 ×100%=6.4%����������ҺŨ��ƫ�ͣ�
×100%=6.4%����������ҺŨ��ƫ�ͣ�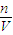 ��֪������Һ��ƫ�ߣ�
��֪������Һ��ƫ�ߣ� ��֪������Һ��Ũ��ƫ�ͣ�
��֪������Һ��Ũ��ƫ�ͣ�

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������100 g 10%��CuSO4��Һ����ȡ10 g CuSO4��������90 gˮ�� �ڲⶨCuSO4�����нᾧˮ�İٷֺ���ʱ�����õľ����Ѿ��ܳ� ������һ�����ʵ���Ũ�ȵ�H2SO4��Һ������ʱ��������ƿ�Ŀ̶��� ���к͵ζ�ʱ���ú�NaOH��KOH���Ƴɵı���Һ�ζ�δ֪Ũ�ȵ�HCl
A.ֻ�Т� B.ֻ�Т� C.�ڢۢ� D.�٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ�����и�һ��ѧ��������У������ѧ�Ծ� ���ͣ�ѡ����
����ʵ������ᵼ��ʵ����ƫ�͵���
��1������100 g10����CuSO4��Һ����ȡ10g������������90 gˮ��
��2������һ�����ʵ�����Ũ�ȵ�H2SO4��Һ��Ũ�����ܽ��δ��ȴ������ת��������ƿ���ж���
��3������һ�����ʵ�����Ũ�ȵ�H2SO4��Һ������ʱ��������ƿ�Ŀ̶���
��4��ʵ������ȡ���������������������ҷ�Ӧ��֣������ռ��������������ʵ����������Ũ���������ʵ����ʵ���
A��ֻ�У�1�� B��ֻ�У�2�� C����2����3����4�� D����1����3����4��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com