
��֪����������ˮ�е��ܽ��Ϊ��0.18g��4�棩��0.34g��25�棩��6.8g��95�棩�����ѵķе�Ϊ34.6�档ʵ���ҳ��ñ���ȩ�Ʊ����״��ͱ����ᣬ��ԭ��Ϊ��2C6H5�DCHO��NaOH C6H5�DCH2OH��C6H5�DCOONa
C6H5�DCH2OH��C6H5�DCOONa
ʵ�鲽�����£�
������ͼ��ʾװ���м������� NaOH��ˮ�ͱ���ȩ�����ȡ����ȣ�ʹ��Ӧ��ֽ��С�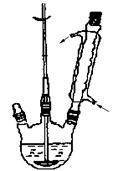
�ڴ��������¿ڼ�����ˮ�����ȣ���ȴ�������Һ©������������ȡ����Һ��ˮ�㱣�����á������Ѳ�������10%̼������Һ��ˮϴ�ӡ�
�۽����Ѳ㵹��ʢ��������ˮ����þ�ĸ�����ƿ�У����ȡ����ú���ת������װ�ã��������ȼ��ȳ�ȥ���ѣ��ռ�198�桫204����ֵñ��״���
�ܽ�������е�ˮ�������Ũ�����Ͼ��ȣ�������ɫ���塣��ȴ�����˵ôֲ�Ʒ�����ֲ�Ʒ�ᴿ�ñ����ᡣ
��1��������У������ˮϴ�ӵ������� ������Һ©��������Һ����뿪��ʵ����������ǣ��� �� ��
��2�����������ˮ����þ�������� ��
��3���������ˮ���Ũ�����Ϻ�����Ӧ�Ļ�ѧ����ʽΪ ������Ӧ��������ȴ��Ŀ���� ��
��4������װ������������������ѹϵͳ�⣬���� �� �����������ƣ���
B����1����ȥNaOH���������ơ�̼���Ƶ����� ��2�֣�
���²�Һ����¶˷ų������ϲ�Һ����Ͽڵ�����ÿ��1�֣���2�֣�
��2���������2�֣�
��3��C6H5�DCOONa��HCl C6H5�DCOOH��NaCl ��2�֣�
C6H5�DCOOH��NaCl ��2�֣�
ʹ�����ᾡ���ܶ������ ��2�֣�
��4�� ����ƿ ��1�֣� ����©����1�֣�
������������������ӻ�������ˮ���ܽ�Ƚϴ���ˮ��ȥNaOH���������ơ�̼���Ƶ����ʣ���Һ©����ʹ�ã����²�Һ����¶˷ų������ϲ�Һ����Ͽڵ������𰸣���ȥNaOH���������ơ�̼���Ƶ����� �����²�Һ����¶˷ų������ϲ�Һ����Ͽڵ���������ˮ����þ����ˮ���MgSO4��7H2O������������𰸣�����������������ữ���ã����������Ʊ�ɱ����ᣬ����Һ����������ȴ��Ŀ����ʹ�����ᾡ���ܶ����������߲��ʣ��𰸣�C6H5�DCOONa��HCl C6H5�DCOOH��NaCl �� ʹ�����ᾡ���ܶ���������ȳ���װ��������ƿ������©������ɣ��𰸣�����ƿ������©����
C6H5�DCOOH��NaCl �� ʹ�����ᾡ���ܶ���������ȳ���װ��������ƿ������©������ɣ��𰸣�����ƿ������©����
���㣺����ѧ���Ի�ѧԭ������ѧʵ�鷽�������ճ̶ȣ���ʵ�鷽���ķ������ۺ����û�ѧ֪ʶ�����ѧʵ���еľ��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʽ��з��ࣺ
��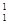 H��
H��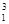 H ��
H ��
��O2��O3��
���Ҵ���C2H5OH������ѣ�CH3��O��CH3�� ��
�������飨CH3CH2 CH2 CH3�����춡�飨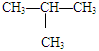 �� ��
�� ��
�ݼ��飨CH4������飨C3H8����
��1����Ϊͬλ�ص��� ������š���ͬ����
��2����Ϊͬϵ����� ��
��3����Ϊͬ���칹����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������һ����;�㷺�ľ�ϸ������Ʒ��ij����С�����ʵ������ȡ���ᴿ���������ķ������£�
��֪�����Ȼ��ƿ����Ҵ��γ�CaCl2��6C2H5OH
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
 CH3CH2OCH2CH3��H2O
CH3CH2OCH2CH3��H2O �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�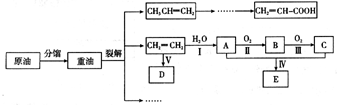
��֪��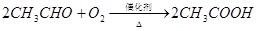
��1����ӦII�Ļ�ѧ����ʽ�� ��
��2��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ�� ��
��3��E������ζ�����ʣ���ʵ��������ͼװ����ȡ��
�ٷ�ӦIV�Ļ�ѧ����ʽ�� ���÷�Ӧ����Ϊ ��
�ڸ�װ��ͼ����һ�����ԵĴ����� ��
��4��Ϊ��֤��Ũ�����ڷ�ӦIV�����˴�������ˮ�������ã�ijͬѧ������ͼ�Ľ���װ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ����ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܼ����Լ� | �Թ������Լ� | �л���ĺ��/cm |
| A | 2 mL�Ҵ���1 mL���ᡢ 1mL18mol��L��1Ũ���� | ����Na2CO3��Һ | 3.0 |
| B | 2 mL�Ҵ���1 mL���� | 0.1 | |
| C | 2 mL�Ҵ���1 mL���ᡢ 3 mL 2mol��L��1 H2SO4 | 0.6 | |
| D | 2 mL�Ҵ���1 mL���ᡢ���� | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪��CH3CH2OH CH2=CH2��+H20
CH2=CH2��+H20
CH2=CH2+Br2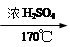 BrCH2��CH2Br
BrCH2��CH2Br
���Ҵ���1,2-�������顢���ѵ��й������������±���ʾ��
| | �Ҵ� | 1,2-�������� | ���� |
| ͨ��״���µ�״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g��cm-3 | 0.79 | 2.2 | 0.71 |
| �۵�/�� | -130 | 9 | -116 |
| �е�/�� | 78.5 | 132 | 34.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ܻ���DBP���ڱ����������������ҪӦ����PVC�Ⱥϳɲ���������������
�ϳɷ�Ӧԭ��Ϊ��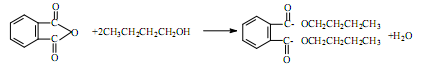
ʵ�鲽�����£�
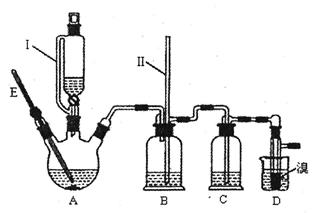
����1����������ƿ�з���14.8g�ڱ�����������25mL��������4��Ũ���ᣬ��������������Ӧװ����ͼ����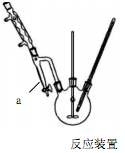
����2�������������ڱ���������������ʧ�����������ڡ�
����3����������һ���̶�ʱ��������150��
����4����ȴ�������©���У��ñ���ʳ��ˮ��5%̼����ϴ�ӡ�
����5����ѹ�����ռ�200~210��2666Pa��֣�����DBP��Ʒ
��1�������������� ��
��2��ͼ������a���Ƽ������� ������3��ȷ���д��������ɵ������� ��
��3���ñ���ʳ��ˮ����ˮϴ�ӵĺô��� ��
��4��̼������Һϴ�ӵ�Ŀ���� ��
��5���ü�ѹ�����Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����һ��˫����ϩ��������ӳɺ�IJ���ṹ��ʽ��ͼ������������еĽṹ�У� ��
| A��4�� | B��5�� | C��6�� | D��7�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����CH4��C2H4�ķ��������
| A���Ƚ�������ˮ�е��ܽ�ȴ�С | B���������ǵ���ζ |
| C��ͨ����ˮ�й۲���Һ��ɫ�ı仯 | D����ȼ���Ǻ۲���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��2�֣��������л���?(CH3)2CHCH(CH3)2��?(CH3)2C(CH3)2��?CH3(CH2)2CH(CH3)2��
?CH3CH2C(CH3)3�йش������ʵ�����������ȷ���� �� ��
| A��?��?��Ϊͬ���칹�壬?��?��Ϊͬϵ�� | B��?��һ�ȴ��������� |
| C��?������ϩ���������ӳɶ���� | D����ͨ�����ⷴӦ�õ�?��Ȳ����3�֡� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com