
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����� ��1����Ҫ220mL 1mol/L��ϡ���ᣬӦ������250mL 1mol/L��ϡ���ᣬ�������ƹ��������ʵ����ʵ�������������Ҫ��Ũ����������
��2������ʵ��������輰������������ѡȡ������
��3������ʵ�������c=$\frac{n}{V}$��Ӱ���жϸ�ѡ���������ҺŨ�ȵ�Ӱ�죻
��4������������0.1mol/LNaOH��Һ450mL��ʵ�������Ƶ���500mL 0.1mol/L����Һ������m=nM=cVM�������Ҫ�������Ƶ�������
�ڸ�������500mL 0.1mol/L��NaOH��Һ�IJ���Ϊ���������ܽ⡢��ȴ����Һ��ϴ����Һ�����ݡ�ҡ�ȵȣ��ݴ˽�������
��� �⣺��1����ʵ���ҽ���Ҫ1mol/L��ϡ����220mL��Ӧ��ѡ�ù��Ϊ250mL������ƿ��������250mL 1mol/L��ϡ���ᣬ����Ҫ��Ũ��������ΪxmL�����ƹ�����������������䣬��98g/mol��1mol/L��0.25L=xmL��1.84g/cm3��98%����ã�x��13.6��
�ʴ�Ϊ��13.6��
��2�����Ʋ����м��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ������ձ���ϡ�ͣ����ò��������裬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ��μ�����Һ������̶���ˮƽ���У��Ǻ�ƿ����ҡ�ȣ�������Ҫ������Ϊ����Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ���ȱ��250mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��3��A����ȡŨ����ʱ�����Ӷ�����������ȡ��Ũ�������ƫС�����Ƶ���Һ�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���A����
B��ϡ��Ũ����ʱ��δ��ȴ�����¼�ת�Ƶ�����ƿ�������ȵ���Һ���ƫ����ȴ��������Һ�����С�����Ƶ���Һ���ƫС����ҺŨ��ƫ�ߣ���B��ȷ��
C��ϴ����ȡŨH2SO4�����Ͳ������ϴ��Һת�Ƶ�����ƿ�У��������Ƶ���Һ�����ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ���C��ȷ��
D������ҡ�Ⱥ���Һ����ڱ��ߣ����ý�ͷ�ιܼ�����ˮ�����ߣ��������Ƶ���Һ���ƫ�����Ƶ���ҺŨ��ƫ�ͣ���D����
E������ƿ��������ڶ��ݻ���Ҫ��������ˮ�����Բ�Ӱ�����ƽ������E����
F������ʱ����������ƿ�̶��ߣ����������ˮ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ���F��ȷ��
�ʴ�Ϊ��BCF��
��4��������0.1mol/LNaOH��Һ450mL��ʵ�������Ƶ���500mL 0.1mol/L����Һ����ҪNaOH�����ʵ���Ϊ��0.1mol/L��0.5L=0.05mol����Ҫ�������Ƶ�����Ϊ��40g/mol��0.05mol=2.0g��
�ʴ�Ϊ��2.0��
�ڲ��������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������ȷ�IJ���˳��Ϊ��BCAFED��
�ʴ�Ϊ��BCAFED��
���� ���⿼������һ�����ʵ���Ũ�ȵ���Һ��������Ŀ�ѶȲ�����ȷ���Ʋ���Ϊ���ؼ���������Ϊ�ѵ㣬��Ҫ����ʵ�������c=$\frac{n}{V}$��Ӱ���жϣ�����֪ʶ��϶࣬��ֿ���ѧ���ķ�����������ѧʵ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 30.5 | B�� | 26.3 | C�� | 26 | D�� | 24 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
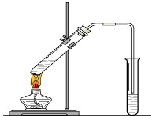 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
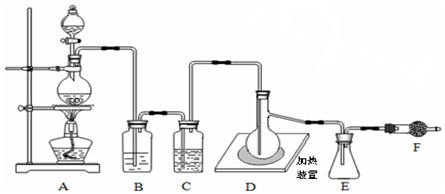
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH ��Һ��CO2 �ķ�Ӧ | B�� | Ba��OH��2 ��Һ��ϡH2SO4 �ķ�Ӧ | ||
| C�� | NaHSO4��Һ��KOH ��Ӧ | D�� | ʯ�����ϡH2SO4 �ķ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 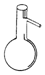 ������ƿ | B�� |  ��Һ©�� | C�� | 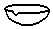 ������ | D�� |  ����ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Mg2+��Cl-��Na+��NO3- | B�� | K+��Na+��NO3-��HCO3- | ||
| C�� | Na+��Ba2+��Cl-��NO3- | D�� | Fe3+��Na+��AlO2-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v��C��=0.5mol/��L•min�� | |
| B�� | x=3 | |
| C�� | B��ת����Ϊ25% | |
| D�� | ��ʹ�ô����������̴ﵽƽ���ʱ�䣬��Aת���ʲ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com