
.��֪���������ʹ���������Һ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��5SO2+2KMnO4+2H2O====K2SO4+2MnSO4+2H2SO4��
��1��Ũ������ľ̿�ڼ��������·�Ӧ�Ļ�ѧ����ʽ��_____________________��
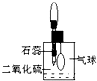
��2��������ͼ���и���װ�����һ��ʵ�飬����֤������Ӧ�������ĸ��ֲ����Щװ�õ�����˳��������������ҵķ�����_________![]() _________
_________![]() _________
_________![]() _________������װ�õı�ţ�
_________������װ�õı�ţ�
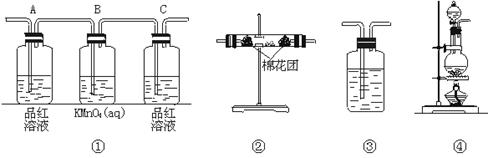
��3��ʵ��ʱ�ɹ۲쵽װ�â���Aƿ����Һ��ɫ��Cƿ����Һ����ɫ��Aƿ��Һ��������_______________��Bƿ��Һ��������______________________��Cƿ��Һ��������_______________��
��4��װ�â����ӵĹ���ҩƷ��_______________������֤�IJ�����_______________��ȷ��װ��������װ����λ�õ�������_______________��
��5��װ�â�����ʢ��Һ��_______________������֤�IJ�����_______________
��1��C+2H2SO4��Ũ��![]() CO2��+2SO2��+2H2O
CO2��+2SO2��+2H2O
��2���� �� �� ��
��3��֤��SO2�Ĵ��� ��ȥSO2 ��֤SO2�Ƿ����
��4����ˮ����ͭ���� ˮ���� ͨ��ˮ��Һ֮ǰ
��5�������ʯ��ˮ CO2
Ũ�����ڼ���������������ľ̿����Ӧ�Ļ�ѧ����ʽΪC+2H2SO4��Ũ��![]() CO2��+2SO2��+2H2O��2����֤���ֲ���ʱ��Ӧ����֤H2O������ˮ����ͭ������֤SO2����Ʒ����Һ���ٳ�ȥSO2������֤CO2����װ�õ�����˳��Ϊ�ܢڢ٢ۣ�3��Aƿ��Һ����������֤SO2�Ĵ��ڡ�Bƿ��Һ�������dz�ȥSO2��Cƿ��Һ����������֤SO2�Ƿ��������4��װ�â�������֤����ˮ�Ĵ��ڣ������ӵĹ���ҩƷΪ��ˮ����ͭ���塣������ǰ������Ϊͨ������װ�ú�����ˮ�֡���5��װ�â�������֤����CO2������ʢ��Һ�dz����ʯ��ˮ��
CO2��+2SO2��+2H2O��2����֤���ֲ���ʱ��Ӧ����֤H2O������ˮ����ͭ������֤SO2����Ʒ����Һ���ٳ�ȥSO2������֤CO2����װ�õ�����˳��Ϊ�ܢڢ٢ۣ�3��Aƿ��Һ����������֤SO2�Ĵ��ڡ�Bƿ��Һ�������dz�ȥSO2��Cƿ��Һ����������֤SO2�Ƿ��������4��װ�â�������֤����ˮ�Ĵ��ڣ������ӵĹ���ҩƷΪ��ˮ����ͭ���塣������ǰ������Ϊͨ������װ�ú�����ˮ�֡���5��װ�â�������֤����CO2������ʢ��Һ�dz����ʯ��ˮ��


 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| X |
| X |
| H2O |
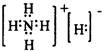
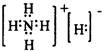
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
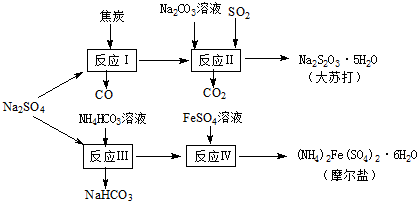 ����Ȼ�κ���Դ�з��������â�������Ṥҵ������������̿��Ϊԭ�ϣ��������մ��Ħ���Σ���ԭ�ϵ��ۺ������ʽϸߣ�����Ҫ�������£�
����Ȼ�κ���Դ�з��������â�������Ṥҵ������������̿��Ϊԭ�ϣ��������մ��Ħ���Σ���ԭ�ϵ��ۺ������ʽϸߣ�����Ҫ�������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
 ��ȡ��ƿ����Һ���������Ʒ����Һ�������ʵ������Ϊ
��ȡ��ƿ����Һ���������Ʒ����Һ�������ʵ������Ϊ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com