
(5��)����������һ�ֽྻ����������Դ����������(��Ҫ�ɷ�Ϊ CO��CO2��H2 ��)�� H2 ��ϣ����ϳɼ״��������������õķ���֮һ��
(1)������Ӧ�Ĵ������� Cu��Zn��Al ��Ԫ�ء�д����̬ Cu2+���ӵĺ�������Ų�ʽ_______________________________________;
(2)���ݵȵ���ԭ����д�� CO ���ӵĽṹʽ______________________;
(3)�״��������ɵõ���ȩ����ȩ������ Cu(OH)2 �ļ�����Һ��Ӧ���� Cu2O ������
�ټ�ȩ������̼ԭ�ӹ�����ӻ�����Ϊ_____________________;
�ڼ�ȩ���ӵĿռ乹����__________________��
�� 1 mol ��ȩ������ �� ������ĿΪ__________________��
��1��1s22s22p63s23p63d9��[Ar]3d9 ��2��
��3����sp2�ӻ� ��ƽ�������� ��3NA��1.806��1024
����������1�����ݹ���ԭ����֪��Cuԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d9��[Ar]3d9��
��2����������ԭ�����ֲ�����ͬ���ǵȵ����壬����CO������ǵȵ����壬��˽ṹʽΪC��O(��C O)��
O)��
��3���ټ�ȩ��ƽ���ͽṹ������̼ԭ����sp2�ӻ���
�ڼ�ȩ��ƽ�������νṹ��
������˫������1���Ҽ���1�� �����ɵģ�����1mol��ȩ�����ЦҼ�����ĿΪ3NA��
�����ɵģ�����1mol��ȩ�����ЦҼ�����ĿΪ3NA��


 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ�ɶ����и߶�10�½��Կ��Ի�ѧ�Ծ����������� ���ͣ������
(5��)����������һ�ֽྻ����������Դ����������(��Ҫ�ɷ�Ϊ CO��CO2��H2��)�� H2��ϣ����ϳɼ״��������������õķ���֮һ��
(1)������Ӧ�Ĵ������� Cu��Zn��Al ��Ԫ�ء�д����̬ Cu2+���ӵĺ�������Ų�ʽ_______________________________________;
(2)���ݵȵ���ԭ����д�� CO ���ӵĽṹʽ______________________;
(3)�״��������ɵõ���ȩ����ȩ������ Cu(OH)2�ļ�����Һ��Ӧ���� Cu2O ������
�ټ�ȩ������̼ԭ�ӹ�����ӻ�����Ϊ_____________________;
�ڼ�ȩ���ӵĿռ乹����__________________��
�� 1 mol ��ȩ������ �� ������ĿΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�콭��������ѧ������ǰ����ѵ����ѧ���� ���ͣ�������
ˮú������Ҫȼ�Ϻͻ���ԭ�ϣ�����ˮ����ͨ�����ȵ�̿���Ƶã�
C (s) + H2O(g) CO (g) +H2 (g) ��H�� +131.3 kJ?mol��1����������������
CO (g) +H2 (g) ��H�� +131.3 kJ?mol��1����������������
��1��ʵ�ʹ�ҵ�����У���̿��������ͨ��ˮ�����Ϳ���������ͨ�������ԭ�������ڸ÷�Ӧ�����ȣ�����̿���¶Ƚ��ͣ��뼰ʱͨ�븻�������ٽ�̿���ȼ�շ��ȣ�
C (s) + O2(g)= CO2 (g)����H = ��393.5kJ��mo1��1 ��������������
Ϊ���������������ԣ������������������IJ�������ģ���ÿ���Ӧͨ���ˮ�����Ϳ���������ȣ�ͬ��ͬѹ��ԼΪ���٣�������������������ռ1/5��
��2��һ���¶��£����������о�������������Ӧ�٣���������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д������Ӧ�Ŀո�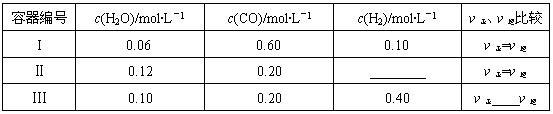
��3�������Ҵ����ɵ��ۻ���ά�ص�������ԭ�Ϸ��ͻ�á������Ҵ��ɽ�����úϳ�����CO��H2�������Ҵ������ϳ�������������·�ߣ�
a��ˮ������������CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g)
b�����ִ�������CH3CH2OH(g)��1/2O2(g)��3H2(g)��2CO(g)
ij���������о����������Ҵ��õ��ĺϳ����ϳ�һ���������͡��Ҵ�����һ�밴a��b��ʽ��Ӧ���ϳ����ϳ��������͵ķ�ӦΪ��2mCO��(2m��n)H2��2CmHn��2mH2O���ٶ��ϳɵ����������к���X��Y���ֳɷ֣���X��Y������8��̼ԭ�ӵ�����X�DZ���ͬϵ�Y��������
��X�ķ���ʽΪ ��Y�ķ���ʽΪ ��
��50����������Ϊ92%���Ҵ�������ת�����ٶ�����ת���ʾ�Ϊ100%���������տɻ��X������Ϊ���ٶ֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ˮú������Ҫȼ�Ϻͻ���ԭ�ϣ�����ˮ����ͨ�����ȵ�̿���Ƶã�
C (s) + H2O(g) ![]() CO (g) +H2 (g) ��H�� +131.3 kJ??mol��1����������������
CO (g) +H2 (g) ��H�� +131.3 kJ??mol��1����������������
��1��ʵ�ʹ�ҵ�����У���̿��������ͨ��ˮ�����Ϳ���������ͨ�������ԭ�������ڸ÷�Ӧ�����ȣ�����̿���¶Ƚ��ͣ��뼰ʱͨ�븻�������ٽ�̿���ȼ�շ��ȣ�
C (s) + O2(g)= CO2 (g)����H = ��393.5kJ��mo1��1 ��������������
Ϊ���������������ԣ������������������IJ�������ģ���ÿ���Ӧͨ���ˮ�����Ϳ���������ȣ�ͬ��ͬѹ��ԼΪ���٣�������������������ռ1/5��
��2��һ���¶��£����������о�������������Ӧ�٣���������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д������Ӧ�Ŀո�

��3�������Ҵ����ɵ��ۻ���ά�ص�������ԭ�Ϸ��ͻ�á������Ҵ��ɽ�����úϳ�����CO��H2�������Ҵ������ϳ�������������·�ߣ�
a��ˮ������������CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g)
b�����ִ�������CH3CH2OH(g)��1/2O2(g)��3H2(g)��2CO(g)
ij���������о����������Ҵ��õ��ĺϳ����ϳ�һ���������͡��Ҵ�����һ�밴a��b��ʽ��Ӧ���ϳ����ϳ��������͵ķ�ӦΪ��2mCO��(2m��n)H2��2CmHn��2mH2O���ٶ��ϳɵ����������к���X��Y���ֳɷ֣���X��Y������8��̼ԭ�ӵ�����X�DZ���ͬϵ�Y��������
��X�ķ���ʽΪ ��Y�ķ���ʽΪ ��
��50����������Ϊ92%���Ҵ�������ת�����ٶ�����ת���ʾ�Ϊ100%���������տɻ��X������Ϊ���ٶ֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com