
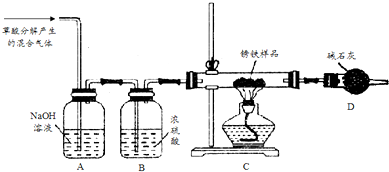
���� ��1����װ��AΪ����������Һ������������Һ�ܹ��������̼��Ӧ����������������Һ����������еĶ�����̼��ȥ��װ��B��ΪŨ���ᣬŨ�����ܹ����ջ�������е�ˮ�֣�
�ڸ���������Ʒ12.6g�����������Ϊ8.4gΪ����������װ��D����8.4gΪ������̼��ˮ�������������������ݿ��Լ����������ɣ�
�۸���һ����̼�ж��������ŷŵ������н��н��
��2��Fe2+���л�ԭ�ԣ���ʹ���������Һ��ɫ���ݴ˿��Լ����������ӣ�
��3������Ũ��������ˮ�ͼ�ʯ������ˮ�Ͷ�����̼�����������̼��������Ȼ����ݶ�����̼������������������Ӷ����x��ֵ���ٸ�����Ʒ�����������m��Fe����m ��Fe2O3•xH2O����
��� �⣺��1���ٸ���ʵ��Ŀ�ģ���Ҫʹ��һ����̼��ԭ���������壬������Ҫ������ֽ����ɵĶ�����̼��ˮ��ȥ��װ��A�е�����������Һ���ڳ�ȥ������̼���壬װ��B�е�Ũ�������ڸ���һ����̼���壬
�ʴ�Ϊ����ȥ��������е�CO2����ȥ��������е�H2O��
�ڼ�����ȫ��Ӧ��õ����������Ϊ8.4gΪ����������װ��D����8.4gΪ������̼��ˮ���������پ�����Ʒ�����������Լ����������ɣ�
�ʴ�Ϊ���ܣ�
��һ����̼�ж��������ŷŵ������У�����Ӧ������β������װ�ã�
�ʴ�Ϊ��ȱ��β������װ�ã�
��2��Fe2+���л�ԭ�ԣ���ʹ���������Һ��ɫ���ݴ˿��Լ����������ӣ�ʵ�����Ϊȡ������Һ�ڽྻ�Թ��У��μӼ������Ը��������Һ����ַ�Ӧ����Һ��ɫ��ȥ��֤������Fe2+������Fe2+��
�ʴ�Ϊ��ȡ������Һ�ڽྻ�Թ��У��μӼ������Ը��������Һ����ַ�Ӧ����Һ��ɫ��ȥ��֤������Fe2+������Fe2+��
��3��Ũ��������ˮ����ʯ������ˮ�Ͷ�����̼��ȡ������Ʒ12.6g����װ��D��Ӳ�ʲ������У�����һ��ʱ������ȫ��Ӧ��õ����������Ϊ8.4g��װ��E����8.4g������װ��E����װŨ�����ϴ��ƿF�����°�������Ʒ�������Ͳ�������ʵ�飬����ȫ��Ӧ��õ������������Ϊ8.4g����װ��F����1.8g������ˮ������Ϊ1.8g��������̼������Ϊ8.4g-1.8g=6.6g����������������Ϊy��
Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2
160 132
y 6.6g
$\frac{160}{y}$=$\frac{132}{6.6}$��
y=8g
����������ˮ�ķ��Ӹ�����=$\frac{8g}{160}$��$\frac{1.8g}{18}$=1��2������x=2��
��Ʒ����������Ϊ��12.6g-��8g+1.8g��=2.8g��������Ʒ�������ʺ�Fe2O3•xH2O��������Ϊ��2.8g����8g+1.8g��=2��7��
�ʴ�Ϊ��2��2��7��
���� ���⿼����̽��������ɼ��������ʺ����ķ�������������������ʵȣ���Ŀ�Ѷ��еȣ�ע������̽��������ɡ��������ʺ������÷�������3��Ϊ������״��㣮


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϵ��ѹǿ���ٷ����仯 | |
| B�� | v��CO2��=v��H2O�� | |
| C�� | ����n mol CH3OH��ͬʱ����n mol H2O | |
| D�� | 3 mol H-H�����ѵ�ͬʱ����2 mol H-O�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | ���� | ��ѧʽ | ��H��kJ•mol-1�� |
| �� | ʯī | C��s�� | -393.5 |
| �� | ���ʯ | C��s�� | -395.4 |
| �� | ���� | H2��g�� | -285.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ȷ | B�� | �ۢ� | C�� | �٢� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ͨ���糵�ȹ���ȡ����Դ������һ����Դ | |
| B�� | �ڼ�������������Դʱ�������ܡ�̫���ܡ����ܽ���Ϊ��Ҫ��Դ | |
| C�� | ��һ��ȷ���Ļ�ѧ��Ӧ�У���Ӧ������������������������һ����ͬ | |
| D�� | ԭ��ؽ��ѻ�ѧ��ֱ��ת��Ϊ���ܣ�������ԭ����ṩ�ĵ�����һ����Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�����ˡ����� | B�� | ���ˡ�����Һ | C�� | ��Һ�������� | D�� | �����ˡ���Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
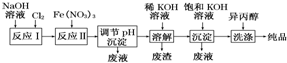
| A�� | ��Ӧ����ҪΪ2NaOH+Cl2�TNaCl+NaClO+H2O ��Ӧ������ӷ���ʽΪ3ClO-+10OH-+2Fe3+�T2FeO42-+3Cl-+5H2O | |
| B�� | ���뱥��KOH��Һ��Ŀ��������K+Ũ�ȣ��ٽ�K2FeO4�������� | |
| C�� | ����pH�����ij���Ϊ�������ƣ��������ϴ�ӵ���ҪĿ���������ڲ�Ʒ���� | |
| D�� | ���������һ�������ˮ���������䴦��ˮ��ԭ��Ϊ���������ǿ�����ԣ���ɱ��������������Fe��OH��3�������ԣ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol•L-1 CH3COOH��Һ�У�c��CH3COO-��+c��OH-��=c��H+�� | |
| B�� | 1 L0��l mol•L-1CuSO4•��NH4��2SO4•6H2O����Һ�У�c��SO42-����c��NH4+����c��Cu2+����c��H+����c��OH-�� | |
| C�� | 0.1mol•L-1NaHCO3��Һ�У�c��Na+��=c��HCO3-��+c��CO32-��+c��H2CO3�� | |
| D�� | ������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ�У�c��Na+����c��X-����c��H+������OH-�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com