
|
��֪��2CH3OH(g)
����˵����ȷ���� | |
| [����] | |
A�� |
ƽ��������¶ȣ�K��400 |
B�� |
ƽ��ʱ��c(CH3OCH3)��1.6 mol/L |
C�� |
ƽ��ʱ����Ӧ����������������20 kJ |
D�� |
ƽ��ʱ���ټ�������ʼ������CH3OH������ƽ���CH3OHת�������� |
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol?L-1�� | 0.44 | 0.6 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol?L-1�� | 0.4 | 0.6 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol?L-1�� | 0.44 | 0.6 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ���� | CH3OH | CH3OCH3 | H2O |
| c/mol?L-1 | 0.44 | 0.60 | 0.60 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
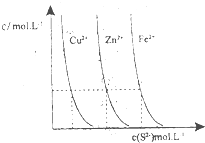 �����ǶԻ�ѧ��Ӧ�仯���̼�������о�����Ҫ��ش����⣺
�����ǶԻ�ѧ��Ӧ�仯���̼�������о�����Ҫ��ش����⣺| ���ữѧʽ | CH3COOH | HCN | H2CO |
| ����ƽ�ⳣ����25�棩 | 1.8��10-5 | 4.9��10-10 | K1=4.3��10-7 K1=5.6��10-11 |
| �ŵ� |
| ͨ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com