
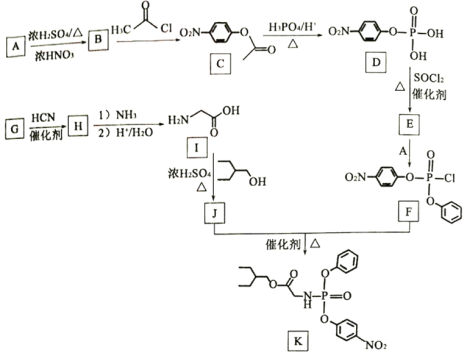
����Ŀ��ҩ�������Τ(Remdesivir)������״������Ⱦ����DZ�ڵ�����Ч����KΪҩ��ϳɵ��м��壬��ϳ�·����ͼ��ʾ��
��֪������Ϣ
��![]()
��
�ش��������⣺
��1��B�Ļ�ѧ����Ϊ____________��
��2��J�к��й����ŵ�����Ϊ____________��
��3����B����C�ķ�Ӧ����Ϊ____________��
��4����G����H�Ļ�ѧ��Ӧ����ʽ____________��
��5��E�к�����Clԭ�ӣ���E�Ľṹ��ʽ____________��
��6��X��C��ͬ���칹�壬д������һ����������������X�Ľṹ��ʽ____________��
�ٱ����Ϻ��������ұ�����ֻ��һ����ԭ�ӣ�
����FeCl3��Һ������ɫ��Ӧ��
��1mol��X����������Na��Ӧ������2gH2��
��7������ɱ��״�(![]() )Ϊԭ�Ϻϳɻ�����
)Ϊԭ�Ϻϳɻ�����![]() ��·��____________(�����Լ���ѡ)��
��·��____________(�����Լ���ѡ)��
���𰸡����������ӣ���4���������ӣ� ���������� ȡ����Ӧ HCHO+HCN![]()
![]() (��
(��![]() + HCN
+ HCN![]()
![]() )
) 
![]()
![]()
![]()
![]()
![]()
![]()
��������
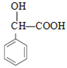
A����ȡ����Ӧ����B��B����ȡ����Ӧ����C������C�ṹ��ʽ֪��BΪ![]() ����AΪ
����AΪ![]() ��D������Ϣ1�ķ�Ӧ����E��E�к�����Clԭ�ӣ���EΪ
��D������Ϣ1�ķ�Ӧ����E��E�к�����Clԭ�ӣ���EΪ ��E��A����ȡ����Ӧ����F��G������Ϣ2�ķ�Ӧ����H��H����ȡ����Ӧ��ˮ�ⷴӦ�õ�I������I�ṹ��ʽ֪HΪHOCH2CN��GΪHCHO��I����������Ӧ����J��F��J����ȡ����Ӧ����K��JΪ
��E��A����ȡ����Ӧ����F��G������Ϣ2�ķ�Ӧ����H��H����ȡ����Ӧ��ˮ�ⷴӦ�õ�I������I�ṹ��ʽ֪HΪHOCH2CN��GΪHCHO��I����������Ӧ����J��F��J����ȡ����Ӧ����K��JΪ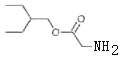 ���ݴ˽��
���ݴ˽��
(1)BΪ![]() ��B�Ļ�ѧ����Ϊ���������ӻ�4�������ӣ�
��B�Ļ�ѧ����Ϊ���������ӻ�4�������ӣ�
(2)JΪ ��J�к��еĹ����ŵ�����Ϊ������������
��J�к��еĹ����ŵ�����Ϊ������������
(3)BΪ![]() ��B����ԭ�ӱ�CH3COȡ������C��B��C�ķ�Ӧ����ȡ����Ӧ��
��B����ԭ�ӱ�CH3COȡ������C��B��C�ķ�Ӧ����ȡ����Ӧ��
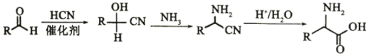
(4)HΪHOCH2CN��GΪHCHO����G����H�Ļ�ѧ��Ӧ����ʽΪHCHO+HCN![]()
![]() (��
(��![]() + HCN
+ HCN![]()
![]() )��
)��
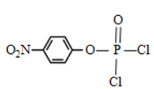
(5)���ݷ�����EΪ ��
��
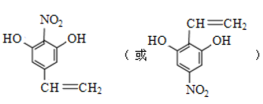
(6)C�Ƕ��������ᱽ����X��Cͬ���칹�壬X�Ľṹ��ʽ���������������ٱ����Ϻ��������ұ�����ֻ��һ����ԭ�ӣ�����FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ�����1mol��X����������Na��Ӧ������2gH2������1mol���������������ǻ������ݲ����Ͷ�֪������CH=CH2���÷��ӽṹ�Գƣ����������Ľṹ��ʽΪ ��
��
(7)�ɱ��״�Ϊԭ���Ʊ�������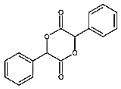 ��
�� ����
����![]() ����������Ӧ�õ���
����������Ӧ�õ���![]() ����
����![]() ����ˮ�ⷴӦ�õ���
����ˮ�ⷴӦ�õ���![]() ���ɱ���ȩ��HCN�����ӳɷ�Ӧ�õ������״�����������Ӧ���ɱ���ȩ����ϳ�·��Ϊ��
���ɱ���ȩ��HCN�����ӳɷ�Ӧ�õ������״�����������Ӧ���ɱ���ȩ����ϳ�·��Ϊ��![]()
![]()
![]()
![]()

![]()

![]()
 ��
��


 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͭ[Cux(Met)y��Met��ʾ�����������]��һ�������������Ӽ���Ϊȷ��������ͭ[Cux(Met)y]����ɣ���������ʵ�飺
(1)��ȡһ����������Ʒ����ƿ�У���������������ˮ��ϡ���ᣬ������ȫ���ܽ⣬��ȴ����Һ�ֳ����ȷݡ�
��ȡ����һ����Һ��������ҺpH��6��8֮�䡣����0.1000 mol/LI2�ı���Һ25.00 mL,��ַ�Ӧ�����2��3��ָʾ��X����0.1000 mol/LNa2S2O3����Һ�ζ�����ɫǡ����ȥ��������Ӧ��![]() ������Na2S2O3����Һ22.00 mL(��������I2��Ӧʱ���ʵ���֮��Ϊ1��1�����ﲻ��Na2S2O3������Ӧ)��
������Na2S2O3����Һ22.00 mL(��������I2��Ӧʱ���ʵ���֮��Ϊ1��1�����ﲻ��Na2S2O3������Ӧ)��
������һ����Һ�м���NH3��H2O-NH4Cl������Һ��������70�����ң�����2-3��ָʾ��PAN����0.02500 mol/LEDTA (Na2H2Y)����Һ�ζ�����Cu2+(���ӷ���ʽΪCu2++H2Y2--=CuY2-+2H+)������EDTA����Һ28.00 mL��
(1)ָʾ��XΪ ____��
(2)��Na2S2O3��Һ�ζ�ʱ����pH��С������S��SO2���ɡ�д��S2O32-��H+��Ӧ�����ӷ���ʽ ___________ ��
(3)���ζ���ˮϴ��δ��EDTA����Һ��ϴ�����Cu2+�����ʵ�����____(�ƫ����ƫС�����䡱)��
(4)ͨ������ȷ��������ͭ[Cux(Met)y]�Ļ�ѧʽ(д���������)________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
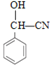
����Ŀ���Է�����AΪԭ�Ϻϳɿ�����ҩ��Prolitane��·������:
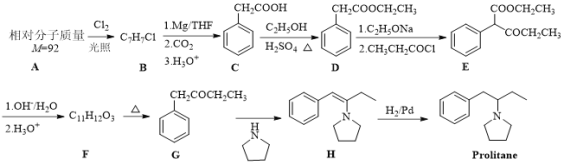
��ش��������⣺
��1��A�Ľṹ��ʽΪ_______��D�Ļ�ѧ����Ϊ_______��
��2��D��E�ķ�Ӧ����Ϊ_______��
��3�� E�Ĺ���������Ϊ_______��
��4��F��G�Ļ�ѧ����ʽΪ_______��
(5) X ��D��ͬϵ���������������X�� ________��(���������칹)
�ٷ�����ɱ�D��һ��CH2���ܷ���������Ӧ�۱����������ֲ�ͬ��ѧ������H�����к˴Ź���������ʾΪ5��壬��������Ϊ3��2��2��2��1��д�����ϸ�Ҫ���X��һ��ͬ���칹��Ľṹ��ʽ��_________________��
��6������Prolitane�ĺϳ�·�ߣ����һ���ɱ����Ҵ�Ϊԭ���Ʊ������������ĺϳ�·��(�������Լ����ܼ���ѡ����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƾ���(Na2S2O3��5H2O)������������������Ҫ��;����֪Na2S2O3��5H2O���������ֽ⣺S2O32����2H����S����SO2����H2O��ij�о�С����ʵ����ģ�ҵ����Ʊ�Na2S2O3��5H2O������ͼ��ʾ��

�������ʵ��װ����ͼ��ʾ��

�ش��������⣺
��1��y����������________________��
��2���ر�K1����K2����Ӧ��ʼ����ʱBװ�õ�������________________��
��3��д��Cװ���з�Ӧ�����ӷ���ʽ________________��
��4��װ��D�������Ǽ���װ��C��SO2������Ч�ʣ�D���Լ���________________������SO2����Ч�ʵ͵�ʵ��������D����Һ________________��
��5��ʵ������ر�K2����K1������Һ���x����ʢҺ�����Ϊ____________(�����)��
A.NaOH��Һ B.Ũ���� C.����NaHSO3��Һ
��6������Һ�л�ý϶�Na2S2O3��5H2O�����ʵ���������Ϊ��________________��________________�����ˣ�ϴ�ӣ����
��7�������ʵ�����Ƶõ�Na2S2O3��5H2O��Ʒ���Ƿ���Na2SO4���ʣ���Ҫ˵��ʵ�����������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ظ����(K2Cr2O7)�������л��ϳɵ��������ʹ����ȡ��ɺ�����Һ(��Ҫ��Cr3����Fe3����K����SO42����)�Ʊ�K2Cr2O7��������ͼ��ʾ��

��֪��I.�����������£�H2O2�ܽ�Cr2O72����ԭΪCr3��
II.��ؽ��������γ��������������pH��Χ��
�������� | ��ʼ������pH | ������ȫ��pH |
Cr3�� | 4.9 | 6.8 |
Fe3�� | 1.5 | 2.8 |
�ش��������⣺
��1�������ڵijɷ���________________��
��2��д���������������з�Ӧ�Ļ�ѧ����ʽ________________��
��3�������ȡ�������Ŀ����________________��
��4������ƽ���ƶ�ԭ�������ữ��pH��1��Ŀ����________________(�����ӷ���ʽ���ʵ�����˵��)��
��5��ͨ������ʵ��ɲⶨ��Ʒ��K2Cr2O7(M��294g/mol)�Ĵ��ȣ���ȡ�ظ��������1.470g����100mL����ƿ���Ƴ���Һ����ȡ25.00mL��Һ�ڵ���ƿ�У���������ϡ����������⻯��(Cr2O72���Ļ�ԭ����ΪCr3��)�����ڰ���5min��Ȼ�����һ������ˮ���������ָʾ������0.1500mol/LNa2S2O3����Һ�ζ��������ı�Һ36.00mL���ζ�ʱ�����ķ�Ӧ�����ӷ���ʽΪ��I2��2S2O32����2I����S4O62���������ⶨ��Ʒ��K2Cr2O7�Ĵ���Ϊ________________��
��6����K2Cr2O7�����£�����������绯ѧ����ʵ�ֺ����ӷ�ˮ����Ч�������乤��ԭ����ͼ��ʾ��
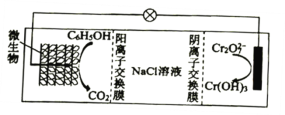
�ٸ����ĵ缫��ӦʽΪ________________��
��һ��ʱ����м���NaCl��Һ��Ũ��________________(�������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȡ1,2���������飬�����Ʊ���������õ���(����)
A. CH3CH2Br![]() CH3CH2OH
CH3CH2OH![]() CH2=CH2
CH2=CH2![]() CH2BrCH2Br
CH2BrCH2Br
B. CH3CH2Br![]() CH2BrCH2Br
CH2BrCH2Br
C. CH3CH2Br![]() CH2=CH2
CH2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH2BrCH2Br
CH2BrCH2Br
D. CH3CH2Br![]() CH2=CH2
CH2=CH2![]() CH2BrCH2Br
CH2BrCH2Br
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�����һ��ԭ��ء��Իش���������(���ݹ��ʺ���)��
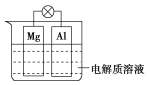
(1)�������ҺΪϡ����ʱ���������� Mg�缫�Ϸ����ķ�ӦΪ____________��Al�缫�Ϸ����ķ�ӦΪ________����Һ��![]() ��________�ƶ�(�Mg�缫����Al�缫��)
��________�ƶ�(�Mg�缫����Al�缫��)
(2)�������ҺΪNaOH��Һʱ������________(��������������������a�⣬���������b��)��_______________
a������������Al�缫Ϊ_______________(�������������)��
b�������ݲ�����������Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ�Ϊ2 L�ĺ����ܱ������г���1 mol CO2(g)��3.5 mol H2(g)����һ�������·�����Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H<0����Ӧ����8 minʱ�ﵽƽ��״̬�����n(CH3OH)=0.5mol���÷�Ӧ��ƽ�ⳣ��K���¶�T�Ĺ�ϵ��ͼ1��ʾ��CO2��ת������ͼ2��ʾ������˵���������
CH3OH(g)+H2O(g) ��H<0����Ӧ����8 minʱ�ﵽƽ��״̬�����n(CH3OH)=0.5mol���÷�Ӧ��ƽ�ⳣ��K���¶�T�Ĺ�ϵ��ͼ1��ʾ��CO2��ת������ͼ2��ʾ������˵���������
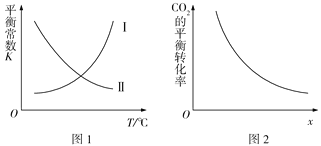
A. ��ͼ1�У����ߢ��ʾ�÷�Ӧ��ƽ�ⳣ��K���¶�T�Ĺ�ϵ
B. ���¶��£�ƽ�ⳣ��K=0.25
C. �������������£�ͼ2��x�ɱ�ʾ�¶Ȼ�ѹǿ��Ͷ�ϱ�c(CO2)/c(H2)
D. �ö�����̼�ϳɼ״�������̼��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС�����������װ�ý���ʵ�飬�ڡ�������Һ���������������������±���
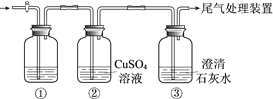
ʵ�� | ���� | ���� |
�� | ��ʢ��Na2S��Һ�Ģ��г���ͨ��CO2������ | ���в�����ɫ��������Һ��pH���ͣ� ���в�����ɫ���ǣ��û�������ð���� |
�� | ��ʢ��NaHCO3��Һ�Ģ��г���ͨ��H2S���������� | ����ͬʵ��� |
���ϣ�CaS��ˮ��ȫˮ��
������ʵ��ó��Ľ�������ȷ����
A. ���а�ɫ������CaCO3
B. ������ҺpH���͵�ԭ���ǣ�H2S+Cu2+ == CuS��+2H+
C. ʵ������CO2���������ķ�Ӧ�ǣ�CO2+H2O+ S2== CO32+ H2S
D. ��ʵ���͢��ܱȽ�H2CO3��H2S���Ե�ǿ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com