
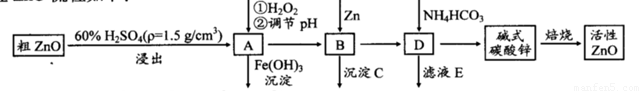
ZnO¾ßÓŠæ¹¾śŠŌ£¬Ņ²ŹĒÖŲŅŖµÄĀÖĢ„Ģķ¼Ó¼Į”£¹¤ŅµÉĻÓÉ“ÖZnO(ŗ¬FeO”¢CuO)Öʱø»īŠŌZnOĮ÷³ĢČēĻĀ£ŗ

ŅŃÖŖ“ĖČÜŅŗÖŠFe2+”¢Fe3+”¢Cu2+”¢Zn2+ŠĪ³ÉĒāŃõ»ÆĪļµÄpHČēĻĀ±ķ£ŗ
| Ąė×Ó | æŖŹ¼³ĮµķµÄpH | ĶźČ«³ĮµķµÄpH |
| Fe2+ | 6.4 | 8.4 |
| Fe3+ | 2.4 | 3.1 |
| Cu2+ | 5.2 | 6.7 |
| Zn2+ | 6.8 | 9 |
(1)ŹµŃéŹŅÖŠÓĆ98£„H2SO4Ą“ÅäÖĘ100 mL60£„Ļ”ĮņĖįĖłŠčŹ¹ÓĆµÄ²£Į§ŅĒĘ÷ÓŠ£ŗ½ŗĶ·µĪ¹Ü”¢ ”¢ ”¢ ”£
(2)Š“³öŌŚAÖŠ¼ÓH2O2µÄĄė×Ó·½³ĢŹ½£ŗ ”£
(3)ĻņAÖŠæÉŅŌ¼ÓČė (Š“»ÆѧŹ½)µ÷½ŚČÜŅŗpH·¶Ī§ŌŚ Ö®¼ä£»³ĮµķCĪŖ ”£
(4)¼īŹ½Ģ¼ĖįŠæ[Zn3(OH)4CO3·H2O]±ŗÉÕÖʱø»īŠŌZnOµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
(5)¼ģŃéSO42-³£ÓĆBaCl2ČÜŅŗ”£³£ĪĀŹ±£¬BaSO4µÄKsp=1.08”Į10-10£¬ĻÖ½«µČĢå»żµÄ BaCl2ČÜŅŗÓė2.0”Į10£3mol/LµÄH2SO4ČÜŅŗ»ģŗĻ”£ČōŅŖÉś³ÉBaSO4³Įµķ£¬ŌBaCl2ČÜŅŗµÄ×īŠ”ÅضČĪŖ____________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÕć½Ź”ŗ¼ÖŻŃ§¾ü֊ѧ2010£2011ѧğø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗ058
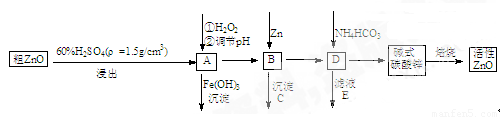
ZnO¾ßÓŠæ¹¾śŠŌ£¬Ņ²ŹĒÖŲŅŖµÄĀÖĢ„Ģķ¼Ó¼Į£®¹¤ŅµÉĻÓÉ“ÖZnO(ŗ¬FeO”¢CuO)Öʱø»īŠŌZnOĮ÷³ĢČēĻĀ£ŗ

ŅŃÖŖ“ĖČÜŅŗÖŠFe2+”¢Fe3+”¢Cu2+”¢Zn2+ŠĪ³ÉĒāŃõ»ÆĪļµÄpHČēĻĀ±ķ

(1)ŹµŃéŹŅÖŠÓĆ98£„””H2SO4Ą“ÅäÖĆĻ”ĮņĖįĖłŠčŹ¹ÓĆµÄ²£Į§ŅĒĘ÷ÓŠ£ŗ½ŗĶ·µĪ¹Ü”¢________”¢________”¢________”£
(2)ŌŚAÖŠ¼ÓH2O2µÄÄæµÄÖ®Ņ»ŹĒŹ¹ČÜŅŗpHÉżøߣ¬Ź¹Fe3+³ĮµķĶźČ«£¬ĮķĶā»¹ÓŠŅ»øöÄæµÄŹĒ________£»ŌŚAÖŠ________(Ģī”°ÄÜ”±»ņ”°²»ÄÜ”±)Ź¹Fe2+Ö±½Ó³Įµķ³żČ„£»
(3)ŅŖŹ¹AČÜŅŗĖ³Ąū³ÉĪŖBČÜŅŗ£¬ČÜŅŗÖŠpHÓ¦æŲÖĘŌŚ________£»
BÖŠ¼ÓČėZn£¬¼ČÄܽµµĶĒāĄė×ÓÅضČÓÖÄÜ________£»
(4)ŹéŠ“¼īŹ½Ģ¼ĖįŠæ±ŗÉÕÖʱø»īŠŌZnOµÄ»Æѧ·½³ĢŹ½________£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ°²»ÕŹ”øßȿ𼶵Ś¶ž“ĪĮŖæ¼Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
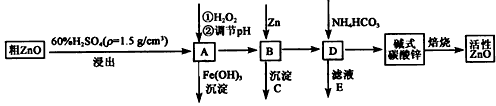
ZnO¾ßÓŠæ¹¾śŠŌ£¬Ņ²ŹĒÖŲŅŖµÄĀÖĢ„Ģķ¼Ó¼Į”£¹¤ŅµÉĻÓÉ“ÖZnO(ŗ¬FeO”¢CuO)Öʱø»īŠŌZnOĮ÷³ĢČēĻĀ£ŗ
ŅŃÖŖ“ĖČÜŅŗÖŠFe2+”¢Fe3+”¢Cu2+”¢Zn2+ŠĪ³ÉĒāŃõ»ÆĪļµÄpHČēĻĀ±ķ£ŗ
|
Ąė×Ó |
æŖŹ¼³ĮµķµÄpH |
ĶźČ«³ĮµķµÄpH |
|
Fe2+ |
6.4 |
8.4 |
|
Fe3+ |
2.4 |
3.1 |
|
Cu2+ |
5.2 |
6.7 |
|
Zn2+ |
6.8 |
9 |
(1)ŹµŃéŹŅÖŠÓĆ98£„H2SO4Ą“ÅäÖĘ100 mL60£„Ļ”ĮņĖįĖłŠčŹ¹ÓĆµÄ²£Į§ŅĒĘ÷ÓŠ£ŗÉÕ±”¢ĮæĶ²”¢ ”¢ ”¢ ”£
(2)Š“³öŌŚAÖŠ¼ÓH2O2µÄĄė×Ó·½³ĢŹ½£ŗ ”£
(3)ĻņAÖŠæÉŅŌ¼ÓČė (Š“»ÆѧŹ½)µ÷½ŚČÜŅŗpH·¶Ī§ŌŚ Ö®¼ä£»³ĮµķCĪŖ ”£
(4)¼īŹ½Ģ¼ĖįŠæ[Zn3(OH)4CO3”¤H2O]±ŗÉÕÖʱø»īŠŌZnOµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğ°²»ÕŹ”øßČżµŚ°Ė“ĪŌĀæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
ZnO¾ßÓŠæ¹¾śŠŌ,Ņ²ŹĒÖŲŅŖµÄĀÖĢ„Ģķ¼Ó¼Į”£¹¤ŅµÉĻÓÉ“ÖZnO£Øŗ¬FeO”¢CuO£©Öʱø»īŠŌZnOĮ÷³ĢČēĻĀ£ŗ
ŅŃÖŖ“ĖČÜŅŗÖŠFe2+ ”¢ Fe3+”¢ Cu2+ ”¢ Zn2+ŠĪ³ÉĒāŃõ»ÆĪļµÄpHČēĻĀ±ķ
|
Ąė×Ó |
æŖŹ¼³Į³ĮµķµÄpH |
ĶźČ«³ĮµķµÄpH |
|
Fe2+ |
6.4 |
8.4 |
|
Fe3+ |
2.4 |
3.1 |
|
Cu2+ |
5.2 |
6.7[Ą“Ō“:Z*xx*k.Com] |
|
Zn2+ |
6.8 |
9 |
£Ø1£©ŹµŃéŹŅÖŠÓĆ98£„H2SO4Ą“ÅäÖĘĻ”ĮņĖįĖłŠčŹ¹ÓĆµÄ²£Į§ŅĒĘ÷ÓŠ£ŗ½ŗĶ·µĪ¹Ü”¢_______”¢_______”¢_______”£
£Ø2£©ŌŚAÖŠ¼ÓH2O2µÄÄæµÄÖ®Ņ»ŹĒŹ¹ČÜŅŗpHÉżøߣ¬Ź¹Fe3+³ĮµķĶźČ«”£ĮķĶā»¹ÓŠŅ»øöÄæµÄŹĒ £»ŌŚAÖŠ (Ģī”±ÄÜ”±»ņ”±²»ÄÜ”±)Ź¹Fe2+Ö±½Ó³Įµķ³żČ„;
£Ø3£©ŅŖŹ¹AČÜŅŗĖ³Ąū³ÉĪŖBČÜŅŗ, ČÜŅŗµÄpHÓ¦æŲÖĘŌŚ ; BÖŠ¼ÓČėZn£¬¼ČÄܽµµĶĒāĄė×ÓÅضČÓÖÄÜ £»
£Ø4£©ŹéŠ“¼īŹ½Ģ¼ĖįŠæ±ŗÉÕÖʱø»īŠŌZnOµÄ»Æѧ·½³ĢŹ½ .
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ°²»ÕŹ”Ä£ÄāĢā ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com