
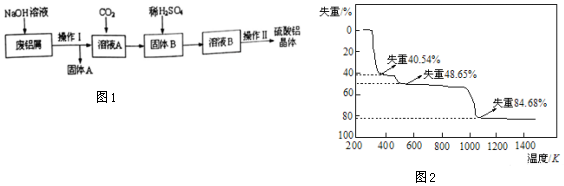
���� �����м�������������м�����������������Һ��������Ӧ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����������Ӧ������AΪFe�����ù��˵ķ������з��룬��ҺAΪNaAlO2��Һ��������ͨ�������̼��������Ӧ��NaAlO2+CO2+2H2O�TAl��OH��3��+NaHCO3����ͨ�����˽��з��룬����BΪ�����������������������ᷴӦ�õ���������Һ���پ�������Ũ������ȴ�ᾧ��ϴ�ӡ�����õ����������壻
��1��Al������������Һ���ɿ����Ե�ƫ�����ƣ�
��2������Һ�л�þ��壬��Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ�����
��3��Al������Ϊ5g-0.95g=4.05g�������������廯ѧʽΪ��Al2��SO4��3��nH2O������AlԪ���غ������������������ʵ������ټ����������������Է�����������������n��ֵ��ȷ����ѧʽ��
��4�����ݣ�3���м����֪�������нᾧˮ���������������¼��ȣ�����ʧȥ�ᾧˮ�������£������������ֽ⣬����ʧ��%�����жϸ��ηֽ�������д��ѧ����ʽ��
��� �⣺��1��Al������������Һ���ɿ����Ե�ƫ��������������þ����Ӧ����Ӧ����ʽΪ2Al+2NaOH+2H2O�T2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����
��2������Һ�л�þ��壬��Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ�����
�ʴ�Ϊ������Ũ�������ˣ�
��3��Al������Ϊ5g-0.95g=4.05g�������ʵ���Ϊ4.05g��27g/mol=0.15mol�������������廯ѧʽΪ��Al2��SO4��3��nH2O������AlԪ���غ㣬��������������ʵ���Ϊ0.15mol��2=0.075mol�����������������Է�������Ϊ49.95��0.075=666����54+96��3+18n=666�����n=18���ʸ�����������Ļ�ѧʽΪ��Al2��SO4��3.18H2O��
�ʴ�Ϊ��Al2��SO4��3.18H2O��
��4�������нᾧˮ�ĺ���Ϊ$\frac{18��18}{666}$=48.65%���ʵڶ�����ȫʧȥ�ᾧˮ���õ�����ΪAl2��SO4��3����һ��ʧȥ���ֽᾧˮ��ʧȥ�ᾧˮ��ĿΪ$\frac{666��40.54%}{18}$=15���ʵ�һ�εõ�������ΪAl2��SO4��3.3H2O����Ӧ����ʽΪ��Al2��SO4��3.18H2O$\frac{\underline{\;\;��\;\;}}{\;}$Al2��SO4��3.3H2O+15H2O��
������ʣ�����ʵ���Է�������Ϊ666����1-84.68%��=102��Ӧ��Al2O3������������Ӧ��������������������Ӧ����ʽΪ��Al2��SO4��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3SO3����
�ʴ�Ϊ��Al2��SO4��3.18H2O$\frac{\underline{\;\;��\;\;}}{\;}$Al2��SO4��3.3H2O+15H2O��Al2��SO4��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3SO3����
���� ���⿼�����ʵ��Ʊ�ʵ�飬Ϊ��Ƶ���㣬�����Ʊ�ʵ�����̼�������ɡ����ʡ������ķ�ӦΪ���Ĺؼ������ط�����ʵ�顢���������Ŀ��飬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ĵ���ʽ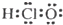 | B�� | ������ĵ���ʽ��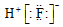 | C�� | OH-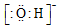 | D�� | 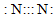 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ʼŨ�ȣ�mol/L�� | �� | �� | �� |
| C��H2�� | 0.010 | 0.020 | 0.020 |
| C��CO2�� | 0.010 | 0.010 | 0.020 |
| A�� | ��Ӧ��ʼʱ�����еķ�Ӧ������죬���еķ�Ӧ�������� | |
| B�� | ƽ��ʱ���ס��ҡ�����CO2��ת���������¹�ϵ���ң���=��=60% | |
| C�� | ƽ��ʱ������c��CO2���Ǽ��е�2������0.012mol/L | |
| D�� | �ı�����ʹ�����¶Ƚ��ͣ���ƽ����H2��Ũ������������Ӧ�ġ�H��0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ�����ܷⱣ�� | |
| B�� | ����ᱣ���ڲ���ƿ�� | |
| C�� | ������ˮ��������ɫ�Լ�ƿ�� | |
| D�� | �ռ���Һ���ô���Ƥ���IJ���ƿ��ʱ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
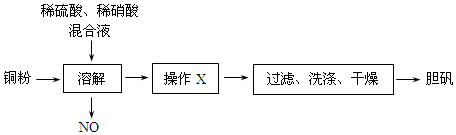
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���Ļ����� | ��ѧ���� |
| A | ����ɻ���ʼ�� | ���ᡱ��������ȼ�ղ�����ˮ |
| B | ������Ϳ��������ɫ��ͭ | �û���Ӧ |
| C | ����»��� | ��Ļ��˳������������ȶ� |
| D | Ұ���ղ��������紵���� | �漰������ԭ��Ӧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ϩ | B�� | ����Ͷ�ϩ | C�� | �������ϩ | D�� | ����Ͷ�ϩ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com