
 A��B��X��Y��Z��ǰ�����ڵij���Ԫ�أ�ԭ��������������A���γ���Ȼ��Ӳ�����ĵ��ʣ�B��Aͬ���ڣ�����������δ�ɶԵ��ӣ�Xԭ�ӵĵ�һ�����������ĵ����ֱܷ��ǣ�I1=578kJ/mol��I2=1817kJ/mol��I3=2745kJ/mol��I4=11575kJ/mol�����³�ѹ�£�Y�����ǹ��壬�����������γ��������Ҫ���ʣ�Z��һ��ͬλ�ص�������Ϊ63��������Ϊ34����ش�
A��B��X��Y��Z��ǰ�����ڵij���Ԫ�أ�ԭ��������������A���γ���Ȼ��Ӳ�����ĵ��ʣ�B��Aͬ���ڣ�����������δ�ɶԵ��ӣ�Xԭ�ӵĵ�һ�����������ĵ����ֱܷ��ǣ�I1=578kJ/mol��I2=1817kJ/mol��I3=2745kJ/mol��I4=11575kJ/mol�����³�ѹ�£�Y�����ǹ��壬�����������γ��������Ҫ���ʣ�Z��һ��ͬλ�ص�������Ϊ63��������Ϊ34����ش����� AԪ�ؿ��γ���Ȼ��Ӳ�����ĵ��ʣ��õ���Ϊ���ʯ����AΪCԪ�أ�B��Aͬ���ڣ�����������δ�ɶԵ��ӣ���BΪNԪ�أ�����Xԭ�ӵĵ�һ�����������ĵ����֪��Xԭ�ӵĵ��ĵ����ܾ�������X����+3�ۣ�����XΪAlԪ�أ����³�ѹ�£�Y�����ǹ��壬�����������γ��������Ҫ���ʣ���YΪSԪ�أ�Z��һ��ͬλ�ص�������Ϊ63��������Ϊ34����������=63-34=29����ZΪCuԪ�أ��ݴ˽��н��
��� �⣺AԪ�ؿ��γ���Ȼ��Ӳ�����ĵ��ʣ��õ���Ϊ���ʯ����AΪCԪ�أ�B��Aͬ���ڣ�����������δ�ɶԵ��ӣ���BΪNԪ�أ�����Xԭ�ӵĵ�һ�����������ĵ����֪��Xԭ�ӵĵ��ĵ����ܾ�������X����+3�ۣ�����XΪAlԪ�أ����³�ѹ�£�Y�����ǹ��壬�����������γ��������Ҫ���ʣ���YΪSԪ�أ�Z��һ��ͬλ�ص�������Ϊ63��������Ϊ34����������=63-34=29����ZΪCuԪ�أ�
��1��AY2��ѧʽ��CS2����ṹʽΪS=C=S��Ϊֱ���ͽṹ�����ڷǼ��Է��ӣ��������ÿ��˫���к���1���Ҽ�������CS2���Ӵ���2���Ҽ���
�ʴ�Ϊ���Ǽ��Է��ӣ�2��
��2��X��AlԪ�أ�����NaOH��Һ��Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2������2Al+2OH-+6H2O=2Al��OH��4+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��3��XB��CN����������ʯ���ƣ�����ԭ�Ӿ��壬�ʴ�Ϊ��ԭ�ӣ�
��4���ǽ�����Խǿ���縺��Խǿ��Al��O��NԪ�صĵ縺����ǽ�����һ�£�������Ԫ�ص縺�Դ�С˳��Ϊ��O��N��Al���ʴ�Ϊ��O��N��Al��
��5��ZΪ29��CuԪ�أ����ݹ���ԭ�������̬ԭ�Ӻ�������Ų�ʽΪ��[Ar]3d104s1��1s22s2 3S23p63d104s1���ʴ�Ϊ��[Ar]3d104s1��?
��6����O2����Ԫ�صĻ��ϼ���0�ۣ�HO2����Ԫ�صĻ��ϼ���-0.5�ۣ����ϼ۽��������������ʢ���ȷ��?
��HO2Ϊ�����ᣬӦ�������ԣ�����Ӧ����HO2�ڼ��в����ȶ����ڣ��ʢ���ȷ��
�ۻ�ԭ������HO2���ʢ۴���
��1molCu�μӷ�Ӧ����+1��ͭ���ӣ���1mol���ӷ���ת�ƣ��ʢ���ȷ��
�������Ϸ�����֪������ȷ��Ϊ�ۣ�
�ʴ�Ϊ���ۣ�
��7��ZΪCuԪ�أ�ͭԭ��λ�ڶ�������ģ�ÿ�������к���ͭԭ�ӵ���ĿΪ��8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�������к���4��ͭԭ�ӣ���������Ϊ��$\frac{64��4}{{N}_{A}}$g����þ����ı߳�Ϊxcm����þ������Ϊ��x3cm3�����ܶ�Ϊ����=$\frac{\frac{64��4}{{N}_{A}}g}{{x}^{3}c{m}^{3}}$=9.00g/cm3�������ɵ�x=$\root{3}{4.72��1{0}^{-23}}$���ʴ�Ϊ��$\root{3}{4.72��1{0}^{-23}}$��
��8��SO42-���ӵ�����ԭ��S�ļ۲���Ӷ�Ϊ��$\frac{6+2}{2}$=4���µ��Ӷ���Ϊ��$\frac{6+2-2��4}{2}$=0������ռ�ṹΪ�������壬Sԭ���ӻ���ʽΪsp3���ʴ�Ϊ��sp3�ӻ���
���� ���⿼����λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�á������ļ��㣬��Ŀ�ѶȽϴ��������Ϣ��ȷ�ƶϸ�Ԫ������Ϊ���ؼ�����7�����漰�ؾ����ļ��㣬Ϊ�ѵ㡢�״��㣬��Ҫ���۽���뼼�ɣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������������������� | |
| B�� | ����������Һ��pH�������� | |
| C�� | ��ʱ����Һ�м���������Ag2O�����ʹ��Һ�ָ����ǰ��״�� | |
| D�� | �����������������������Ϊ2��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 18��18 | B�� | 18g��18g | C�� | 18g/mol��18g | D�� | 18g��18g/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
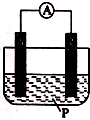 ��ͼ��ʾװ���У��ɹ۲쵽������ָ��ƫת��M����֣�N����ϸ���ɴ��ж��±�����M��N��P���ʣ����п��Գ������� ��������
��ͼ��ʾװ���У��ɹ۲쵽������ָ��ƫת��M����֣�N����ϸ���ɴ��ж��±�����M��N��P���ʣ����п��Գ������� ��������| ѡ�� | M | N | P |
| A | п | ͭ | ϡ������Һ |
| B | ͭ | п | ϡ���� |
| C | �� | п | ��������Һ |
| D | п | �� | ��������Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
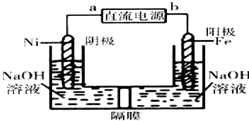 ���û�ѧԭ��֪ʶ�о���ѧ�������������������������ش�
���û�ѧԭ��֪ʶ�о���ѧ�������������������������ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ϊ7��������Ϊ8�ĵ�ԭ�ӣ�${\;}_{7}^{8}$N | |
| B�� | �廯淋ĵ���ʽ�� | |
| C�� | ��ԭ�ӵĽṹʾ��ͼ�� | |
| D�� | �������ױ��Ľṹ��ʽ��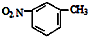 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ⱥ��ʳ��ˮʱ�������ĵ缫��ӦʽΪ��2Cl--2e-�TCl2�� | |
| B�� | ����ȼ�ϵ�صĸ�����Ӧʽ��O2+4H++4e-�T2H2O | |
| C�� | ��ͭ����ʱ�����Դ���������Ǵ�ͭ���缫��ӦʽΪCu-2e-�TCu2+ | |
| D�� | ���������绯ѧ��ʴ��������Ӧʽ��Fe-2e-�TFe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
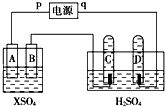 ��ͼ��p��qΪֱ����Դ��������A��+2�۽�������X�Ƴɣ�B��C��DΪ���缫����ͨ��Դ������X������B����ͬʱC��D���Ͼ��������ݣ��Իش�
��ͼ��p��qΪֱ����Դ��������A��+2�۽�������X�Ƴɣ�B��C��DΪ���缫����ͨ��Դ������X������B����ͬʱC��D���Ͼ��������ݣ��Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ±�ص��ʵ���������������7 | |
| B�� | ±�ص�����H2���ϵ����׳̶�ΪF2��Cl2��Br2��I2 | |
| C�� | ±�ص�����H2���ϵIJ������������ȶ��Լ�������ˮ��Һ��������ǿ | |
| D�� | ��F��I��Ԫ�طǽ��������μ�������������������ˮ����������μ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com