
£Ø 8·Ö£© ŅŃÖŖ£ŗŌŚ298K”¢100kPaŹ±£¬
¢ŁC(s,ŹÆÄ«)£«O2(g)= CO2(g) ”÷H1 = £400 kJ”¤mol£1£»
¢Ś2H2(g)£«O2(g) = 2H2O(l) ”÷H2 = £570 kJ”¤mol£1£»
¢Ū2C2H2(g)£«5O2(g) = 4CO2(g)+2H2O(l) ”÷H3 = £2600 kJ”¤mol£1£»
£Ø1£©Š“³ö298KŹ±ÓÉC(s,ŹÆÄ«)ŗĶH2(g)Éś³É1 mol C2H2(g)·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ĻÖÓŠŅ»¶ØĮæµÄŅŅČ²ŌŚ×ćĮæŃõĘųÖŠĶźČ«Č¼ÉÕ£¬·Å³öČČĮæ650 kJ”£½«·“Ó¦ŗóµÄ¶žŃõ»ÆĢ¼ĘųĢå»ŗ»ŗĶØČėµ½ŗ¬ÓŠ0.5 mol Ca (OH)2µÄ³ĪĒåŹÆ»ŅĖ®ÖŠ³ä·Ö·“Ó¦”£ĖłµĆČÜŅŗĪŖ ”£
½«·“Ó¦ŗóµÄČÜŅŗ·ÖĪŖa”¢bĮ½µČ·Ż£¬·Ö±š½ųŠŠĻĀĮŠŹµŃ飬»Ų“šĻąÓ¦ĪŹĢā£ŗ
¢ŁŌŚaÖŠ¼ÓČėÉŁĮæµÄĒāŃõ»ÆÄĘČÜŅŗ£¬Š“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
¢Ś¼ÓČČb£¬¹Ū²ģµ½ ”£
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø 8·Ö£©ŅŃÖŖ°±Ė®µÄµēĄė¶ČÓė“×ĖįµÄµēĄė¶ČŌŚĶ¬ĪĀĶ¬ÅضČĻĀĻąµČ£¬ČÜÓŠŅ»¶ØĮæ°±µÄĀČ»Æļ§ČÜŅŗ³Ź¼īŠŌ”£ĻÖĻņÉŁĮæµÄMg(OH)2Šü×ĒŅŗÖŠ£¬¼ÓČėŹŹĮæµÄ±„ŗĶĀČ»Æļ§ČÜŅŗ£¬¹ĢĢåĶźČ«Čܽā”£
¼×Ķ¬Ń§µÄ½āŹĶŹĒ£ŗMg(OH)2£Ø¹Ģ£©Mg2£«£Øaq£©+2OH”Ŗ£Øaq£©””””¢Ł
NH4£«+H2ONH3”¤H2O +H£«””””¢Ś H£«+OHØD
H2O””””¢Ū
ÓÉÓŚNH4£«Ė®½āĻŌĖįŠŌ£¬H£«ÓėOHØD·“Ӧɜ³ÉĖ®£¬µ¼ÖĀ·“Ó¦¢ŁĘ½ŗāÓŅŅĘ£¬³ĮµķČܽā£»
ŅŅĶ¬Ń§µÄ½āŹĶŹĒ£ŗMg(OH)2£Ø¹Ģ£©Mg2£«£Øaq£©+2OH”Ŗ£Øaq£©””¢Ł NH4£«+OHØD
NH3”¤H2O””¢Ś
ÓÉÓŚNH4ClµēĄė³öµÄNH4£«ÓėMg(OH)2µēĄė³öµÄOHØD½įŗĻ£¬Éś³ÉĮĖČõµÄµē½āÖŹNH3”¤H2O£¬µ¼ÖĀ·“Ó¦¢ŁµÄĘ½ŗāÓŅŅĘ£¬Mg(OH)2³ĮµķČܽā”£
£Ø1£©±ūĶ¬Ń§²»ÄÜæĻ¶ØÄÄĪ»Ķ¬Ń§µÄ½āŹĶŗĻĄķ£¬ÓŚŹĒŃ”ÓĆĻĀĮŠµÄŅ»ÖÖŹŌ¼Į£¬Ą“Ö¤Ć÷¼×”¢ŅŅĮ½Ī»Ķ¬Ń§µÄ½āŹĶÖ»ÓŠŅ»ÖÖÕżČ·£¬ĖūŃ”ÓƵďŌ¼ĮŹĒ £ØĢīŠ“±ąŗÅ£©”£
A.NH4NO3 B.CH3COONH4 C.Na2CO3 D.NH3”¤H2O
£Ø2£©ĒėÄćĖµĆ÷±ūĶ¬Ń§×÷³öøĆŃ”ŌńµÄĄķÓÉ ”£
£Ø3£©±ūĶ¬Ń§½«ĖłŃ”ŹŌ¼ĮµĪČėMg(OH)2µÄ×ĒŅŗÖŠ£¬Mg(OH)2Čܽā£»ÓÉ“ĖĶĘÖŖ£¬¼×ŗĶŅŅÄÄĪ»Ķ¬Ń§µÄ½āŹĶøüŗĻĄķ £ØĢī”°¼×”±»ņ”°ŅŅ”±£©£»Ķź³ÉNH4Cl±„ŗĶČÜŅŗŹ¹Mg(OH)2Šü×ĒŅŗČܽāµÄĄė×Ó·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½¶«Ź”Ū²³ĒĻŲµŚŅ»ÖŠŃ§ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø 8·Ö£© ŅŃÖŖ£ŗŌŚ298K”¢100kPaŹ±£¬
¢ŁC(s,ŹÆÄ«)£«O2(g) = CO2(g) ”÷H1 = £400 kJ”¤mol£1£»
¢Ś2H2(g)£«O2(g) = 2H2O(l) ”÷H2 = £570 kJ”¤mol£1£»
¢Ū2C2H2(g)£«5O2(g) = 4CO2(g)+ 2H2O(l) ”÷H3 = £2600 kJ”¤mol£1£»
£Ø1£©Š“³ö298KŹ±ÓÉC(s,ŹÆÄ«)ŗĶH2(g)Éś³É1 mol C2H2(g)·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ĻÖÓŠŅ»¶ØĮæµÄŅŅČ²ŌŚ×ćĮæŃõĘųÖŠ ĶźČ«Č¼ÉÕ£¬·Å³öČČĮæ650 kJ”£½«·“Ó¦ŗóµÄ¶žŃõ»ÆĢ¼ĘųĢå»ŗ»ŗĶØČėµ½ŗ¬ÓŠ0.5 mol Ca (OH)2µÄ³ĪĒåŹÆ»ŅĖ®ÖŠ³ä·Ö·“Ó¦”£ĖłµĆČÜŅŗĪŖ ”£
ĶźČ«Č¼ÉÕ£¬·Å³öČČĮæ650 kJ”£½«·“Ó¦ŗóµÄ¶žŃõ»ÆĢ¼ĘųĢå»ŗ»ŗĶØČėµ½ŗ¬ÓŠ0.5 mol Ca (OH)2µÄ³ĪĒåŹÆ»ŅĖ®ÖŠ³ä·Ö·“Ó¦”£ĖłµĆČÜŅŗĪŖ ”£
½«·“Ó¦ŗóµÄČÜŅŗ·ÖĪŖa”¢bĮ½µČ·Ż£¬·Ö±š½ųŠŠĻĀĮŠŹµŃ飬»Ų“šĻąÓ¦ĪŹĢā£ŗ
¢ŁŌŚaÖŠ¼ÓČėÉŁĮæµÄĒāŃõ»ÆÄĘČÜŅŗ£¬Š“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
¢Ś¼ÓČČb£¬¹Ū²ģµ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģɽ¶«Ź”¼ĆÄžŹŠĮŗɽ¶žÖŠøßČż12ŌĀŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø 8·Ö£©ŅŃÖŖ°±Ė®µÄµēĄė¶ČÓė“×ĖįµÄµēĄė¶ČŌŚĶ¬ĪĀĶ¬ÅضČĻĀĻąµČ£¬ČÜÓŠŅ»¶ØĮæ°±µÄĀČ»Æļ§ČÜŅŗ³Ź¼īŠŌ”£ĻÖĻņÉŁĮæµÄMg(OH)2Šü×ĒŅŗÖŠ£¬¼ÓČėŹŹĮæµÄ±„ŗĶĀČ»Æļ§ČÜŅŗ£¬¹ĢĢåĶźČ«Čܽā”£
¼×Ķ¬Ń§µÄ½āŹĶŹĒ£ŗMg(OH)2£Ø¹Ģ£©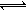 Mg2£«£Øaq£©+2OH”Ŗ£Øaq£©””””¢Ł
Mg2£«£Øaq£©+2OH”Ŗ£Øaq£©””””¢Ł
NH4£«+H2O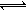 NH3”¤H2O +H£«””””¢Ś H£«+OHØD
NH3”¤H2O +H£«””””¢Ś H£«+OHØD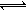 H2O””””¢Ū
H2O””””¢Ū
ÓÉÓŚNH4£«Ė®½āĻŌĖįŠŌ£¬H£«ÓėOHØD·“Ӧɜ³ÉĖ®£¬µ¼ÖĀ·“Ó¦¢ŁĘ½ŗāÓŅŅĘ£¬³ĮµķČܽā£»
ŅŅĶ¬Ń§µÄ½āŹĶŹĒ£ŗMg(OH)2£Ø¹Ģ£©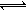 Mg2£«£Øaq£©+2OH”Ŗ£Øaq£©””¢Ł NH4£«+OHØD
Mg2£«£Øaq£©+2OH”Ŗ£Øaq£©””¢Ł NH4£«+OHØD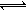 NH3”¤H2O””¢Ś
NH3”¤H2O””¢Ś
ÓÉÓŚNH4ClµēĄė³öµÄNH4£«ÓėMg(OH)2µēĄė³öµÄOHØD½įŗĻ£¬Éś³ÉĮĖČõµÄµē½āÖŹNH3”¤H2O£¬µ¼ÖĀ·“Ó¦¢ŁµÄĘ½ŗāÓŅŅĘ£¬Mg(OH)2³ĮµķČܽā”£
£Ø1£©±ūĶ¬Ń§²»ÄÜæĻ¶ØÄÄĪ»Ķ¬Ń§µÄ½āŹĶŗĻĄķ£¬ÓŚŹĒŃ”ÓĆĻĀĮŠµÄŅ»ÖÖŹŌ¼Į£¬Ą“Ö¤Ć÷¼×”¢ŅŅĮ½Ī»Ķ¬Ń§µÄ½āŹĶÖ»ÓŠŅ»ÖÖÕżČ·£¬ĖūŃ”ÓƵďŌ¼ĮŹĒ £ØĢīŠ“±ąŗÅ£©”£
| A£®NH4NO3 | B£®CH3COONH4 | C£®Na2CO3 | D£®NH3”¤H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģɽ¶«Ź”ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø 8·Ö£© ŅŃÖŖ£ŗŌŚ298K”¢100kPaŹ±£¬
¢ŁC(s,ŹÆÄ«)£«O2(g) = CO2(g) ”÷H1 = £400 kJ”¤mol£1£»
¢Ś2H2(g)£«O2(g) = 2H2O(l) ”÷H2 = £570 kJ”¤mol£1£»
¢Ū2C2H2(g)£«5O2(g) = 4CO2(g)+ 2H2O(l) ”÷H3 = £2600 kJ”¤mol£1£»
£Ø1£©Š“³ö298KŹ±ÓÉC(s,ŹÆÄ«)ŗĶH2(g)Éś³É1 mol C2H2(g)·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ĻÖÓŠŅ»¶ØĮæµÄŅŅČ²ŌŚ×ćĮæŃõĘųÖŠĶźČ«Č¼ÉÕ£¬·Å³öČČĮæ650 kJ”£½«·“Ó¦ŗóµÄ¶žŃõ»ÆĢ¼ĘųĢå»ŗ»ŗĶØČėµ½ŗ¬ÓŠ0.5 mol Ca (OH)2µÄ³ĪĒåŹÆ»ŅĖ®ÖŠ³ä·Ö·“Ó¦”£ĖłµĆČÜŅŗĪŖ ”£
½«·“Ó¦ŗóµÄČÜŅŗ·ÖĪŖa”¢bĮ½µČ·Ż£¬·Ö±š½ųŠŠĻĀĮŠŹµŃ飬»Ų“šĻąÓ¦ĪŹĢā£ŗ
¢ŁŌŚaÖŠ¼ÓČėÉŁĮæµÄĒāŃõ»ÆÄĘČÜŅŗ£¬Š“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
¢Ś¼ÓČČb£¬¹Ū²ģµ½ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com