
| �ζ����� | ������� | NaOH��Һ���������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 18.10 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ۢޢ� | B���٢ۢݢ� | C���ݢ� | D���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
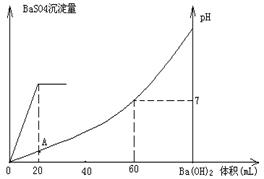
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ���� | ����Һ��� (mL) | �����������Һ�����(mL) | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.50 | 20.70 |
| 2 | 20.00 | 6.00 | 26.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ����Ʒ | ��Һ�¶� | �к��ȡ�H | |||
| t1 | t2 | ||||
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.3�� | |
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.5�� | |
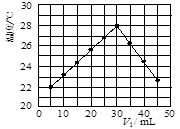
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
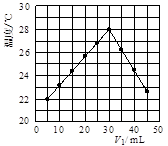
| A������ʵ��ʱ�����¶�Ϊ22 �� |
| B����ʵ�������ѧ�ܿ���ת��Ϊ���� |
| C��NaOH��Һ��Ũ��Լ��1.00 mol/L |
| D����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��10-2 mol/L | B��10-12 mol/L | C��2��10-12 mol/L | D��0.02mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �¶� | v(H2C2O4) | v(KMnO4) | KMnO4��ɫʱ�� |
| 40 �� | 10 ml | 10 ml | 40 s |
| 40 �� | 20 ml | 20 ml | |
 H++In�������������In������ɫ�� �����ȵ������������ȣ� ����ǰ��ǿ��ǰ������ʮ�ֽӽ������жϣ���
H++In�������������In������ɫ�� �����ȵ������������ȣ� ����ǰ��ǿ��ǰ������ʮ�ֽӽ������жϣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.1 mol/L��ˮ�У�c(NH4��) + c(H��) = c(OH��) |
| B��0.1 mol/L��NH4Cl��Һ�У�c(NH4��) = c(Cl��) |
| C��ͬΪ0.1 mol/L������Ͱ�ˮ�������Ϻ�c(NH4��) + 2c(NH3��H2O) = 2c(SO42��) |
| D��pH=3�������pH=11�İ�ˮ�������Ϻ�c(OH��) = c(H��) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com