
���չ���֪��ɽ����Ʒ�ơ���Ʋ��͡������ý���ع⺬�°��ﱽ��(a)�ų��ꡣ����(a)����һ���°��
�»�ԭ���ձ����Ҳ�Ƕ�����ж�������һ��ǿ�°������(a)�ŵĽṹ��ʽ��ͼ��ʾ�������йر�
��(a)�ŵ�˵���в���ȷ���� (����)
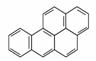 A������(a)�ŵķ���ʽΪC20H12�����ڳ���������
A������(a)�ŵķ���ʽΪC20H12�����ڳ���������
B������(a)���� ��Ϊͬ���칹��
��Ϊͬ���칹��
C������(a)����һ�������¿��Է���ȡ����Ӧ��������ʹ����KMnO4��Һ��ɫ
D������(a)�Ų� ������ˮ�������ڱ����ȷµ��л��ܼ�
������ˮ�������ڱ����ȷµ��л��ܼ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
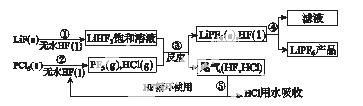
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
(1)�ڢٲ���Ӧ����ˮHF��������________________��________________����Ӧ�豸�����ò������ʵ�ԭ����______________________________________________(�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%��________��Һ��ϴ��
(2)������������ˮ �����½��У��ڢ۲���Ӧ��PF5����ˮ�⣬�����Ϊ�����ᣬд��PF5ˮ��Ļ�ѧ����ʽ��____________________________________��
�����½��У��ڢ۲���Ӧ��PF5����ˮ�⣬�����Ϊ�����ᣬд��PF5ˮ��Ļ�ѧ����ʽ��____________________________________��
(3)�ڢܲ�������õķ�����________���ڢݲ�����β����HF��HCl���õķ�����________��
(4)LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒw g�����Li�����ʵ���Ϊn mol�������Ʒ��LiPF6�����ʵ���Ϊ________mol(�ú�w��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
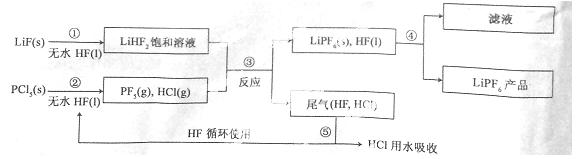
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
��1���ڢٲ���Ӧ����ˮHF�������� �� ����Ӧ�豸�����ò������ʵ�ԭ���� (�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%�� ��Һ��ϴ��
��2��������������ˮ�����½��У��ڢ۲���Ӧ��PCl5����ˮ�⣬�����Ϊ�����ᣬд��PF5ˮ��Ļ�ѧ����ʽ�� ��
��3���ڢܲ�������õķ����� ���ڢݲ�����β����HF��HCl���õķ����� ��
��4��LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒwg�����Li�����ʵ���Ϊnmol�������Ʒ��LiPF6�����ʵ���Ϊ mol(�ú���w��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧʵ����ͨ���ô�п��ϡ���ᷴӦ������������������Һ�к��д���������п��ͬʱ�����ڴ�п�л������������ʣ�ʹ����Һ�л���һ����������������Ϊ�˳�����������Һ���������Ʊ�𩷯(ZnSO4·7H2O)��ijУ��ѧ��ȤС���ͬѧ���������ķ�ҺΪԭ������ȡ𩷯���Ʊ�𩷯��ʵ����������ͼ��ʾ��

��֪����ʼ�����������������������ȫ��pH��Χ�ֱ�ΪFe(OH)3��2.7��3.7��Fe(OH)2��7.6��9.6��Zn(OH)2��5.7��8.0���Իش��������⣺
(1)������Լ��٣���ѡ��ʹ�õ��У���ˮ��NaClO��Һ��20%��H2O2��Ũ���ᡢŨ����ȣ����ѡ��________����������
______________________________________________________________________��
(2)������Լ��ڣ���ѡ��ʹ�õ��У�a.Zn�ۡ�b.ZnO��c.Zn(OH)2��d.ZnCO3��e.ZnSO4�ȣ���ѡ��__________��
(3)�Ӿ���1������2���ù��̵�������__________��
(4)�ڵõ�𩷯ʱ�������м��������ƾ�ϴ�Ӷ�����ˮ��ԭ����_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ����ȡ����Ӧ����(����)
A����ϩͨ�����Ը��������Һ��
B����ϩͨ����ˮ��
C�������������������£�����������Ӧ
D������Һ���Ϻ�� ������
������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ϩ��һ��ʳ�����ϣ���ṹ��ʽ��ͼ��ʾ�������й�����ϩ�ķ�����ȷ����(����)
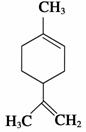 A������һ�ȴ�����6��
A������һ�ȴ�����6��
B�����ķ��������е�̼ԭ��һ����ͬһƽ����
C�����Ͷ�����(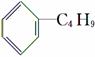 )��Ϊͬ���칹��
)��Ϊͬ���칹��
D��һ�������£����ֱ���Է����ӳɡ�ȡ������������ԭ�ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�������Ļ�����A������Է�������Ϊ104��̼����������Ϊ92.3%.
��1��A�ķ���ʽΪ_____________________________________________________��
��2��A��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽΪ___________________����Ӧ������________��
��3����֪

��д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ�Ļ�ѧ����ʽ__________________________��
��4����һ�������£�A��������Ӧ���õ��Ļ�������̼����������Ϊ85.7%.д���˻�����Ľṹ��ʽ____________________________________________________________��
 ��5����һ�������£���A�ۺϵõ��ĸ߷��ӻ�����Ľṹ��ʽΪ________________��
��5����һ�������£���A�ۺϵõ��ĸ߷��ӻ�����Ľṹ��ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ŀ����������������������������ıȽϣ���ȷ����
���������������������壻�ڶ�����̼���������������壻��ˮ�������������������塣
A���ڢ� B���٢� C���٢� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������غͶ������̵Ļ����60g�����������岻�ټ���ʱ��ʣ�����ʵ�����Ϊ40.8g����㣨1������������������
��2��ԭ�����������ص�������
��3��ʣ��������Ȼ��ص��������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com