
·ÖĪö £Ø1£©ÅØĮņĖįµÄÖŹĮæŌö¼ÓĮĖ3.6g£¬¼“Éś³ÉĖ®µÄÖŹĮæŹĒ3.6g£¬Ķعż×ćĮæ³ĪĒåŹÆ»ŅĖ®£¬æÉµĆ¾øÉŌļŗóµÄ³Įµķ10g£¬¾Ż“ĖæÉŅŌ¼ĘĖćÉś³É¶žŃõ»ÆĢ¼µÄÖŹĮ棬Ź£ÓąĘųĢåŅ»Ńõ»ÆĢ¼æÉŅŌŗĶ×ĘČȵÄŃõ»ÆĢś³ä·Ö·“Ó¦£¬Éś³É½šŹōĢśŗĶ¶žŃõ»ÆĢ¼£¬ŌŁĶØČė³ĪĒåŹÆ»ŅĖ®Ź±£¬æÉøł¾ŻÉś³É³ĮµķµÄÖŹĮæ»ńµĆÉś³É¶žŃõ»ÆĢ¼µÄÖŹĮ棬½ų¶ųĒó³öŅ»Ńõ»ÆĢ¼µÄÖŹĮ棬øł¾ŻŌŖĖŲŹŲŗćæÉŅŌ¼ĘĖćÓŠ»śĪļÖŹµÄ·Ö×ÓŹ½£»
£Ø2£©øł¾ŻøĆÓŠ»śĪļ¾ßÓŠµÄŠŌÖŹ¼°·Ö×ÓŹ½Č·¶ØĘä½į¹¹¼ņŹ½£®
½ā“š ½ā£ŗ£Ø1£©±ź×¼×“æöĻĀ4.48LŃõĘųµÄĪļÖŹµÄĮæĪŖ£ŗ$\frac{4.48L}{22.4L/mol}$=0.2mol£¬
0.1molĢžµÄŃÜÉśĪļŗĶ0.2molŃõĘų·“Ó¦£¬Éś³ÉĖ®µÄÖŹĮæŹĒ3.6g£¬Éś³ÉĖ®µÄĪļÖŹµÄĮæŹĒ£ŗ$\frac{3.6g}{18g/mol}$=0.2mol£¬ĖłŅŌÓŠ»śĪļÖŠHµÄĪļÖŹµÄĮæĪŖ0.4mol£¬Ķعż×ćĮæ³ĪĒåŹÆ»ŅĖ®£¬æɵĆøÉŌļŗóµÄ³Įµķ10g£¬øł¾ŻCO2”«CaCO3æÉÖŖ¶žŃõ»ÆĢ¼µÄĪļÖŹµÄĮæŹĒ£ŗ$\frac{10g}{100g/mol}$=0.1mol£¬Ņ»Ńõ»ÆĢ¼Óė×ĘČȵÄŃõ»ÆĢś³ä·Ö·“Ó¦ŗóŌŁĶØČė³ĪĒåŹÆ»ŅĖ®Ź±£¬æÉµĆ¾øÉŌļŗóµÄ³Įµķ20g£¬øł¾ŻCO”«CO2”«CaCO3æÉÖŖÉś³ÉŅ»Ńõ»ÆĢ¼µÄĪļÖŹµÄĮæŹĒ£ŗ$\frac{20g}{100g/mol}$=0.2mol£¬ŌņÓŠ»śĪļÖŠŗ¬CµÄĪļÖŹµÄĮæŹĒ£ŗ0.1mol+0.2mol=0.3mol£¬
øł¾ŻŃõŌ×ÓŹŲŗć£¬ŗ¬ÓŠŃõŌ×ÓµÄĪļÖŹµÄĮæĪŖ£ŗ0.2mol+0.1mol”Į2+0.2mol-0.2mol”Į2=0.2mol£¬
ĖłŅŌÓŠ»śĪļÖŠŗ¬ÓŠC”¢H”¢OµÄŌ×ÓøöŹż·Ö±šĪŖ£ŗN£ØC£©=$\frac{0.3mol}{0.1mol}$=3”¢N£ØH£©=$\frac{0.4mol}{0.1mol}$=4”¢n£ØO£©=$\frac{0.2mol}{0.1mol}$=2£¬ŌņøĆÓŠ»śĪļµÄ·Ö×ÓŹ½ĪŖ£ŗC3H4O2£¬
“š£ŗøĆÓŠ»śĪļµÄ»ÆѧŹ½ĪŖC3H4O2£»
£Ø2£©ÓŠ»śĪļæÉÓė“¼·¢Éśõ„»Æ·“Ó¦£¬Ōņŗ¬ÓŠōČ»ł£¬ĒŅæÉŹ¹äåĖ®ĶŹÉ«£¬ŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬ĖłŅŌÓŠ»śĪļµÄ½į¹¹¼ņŹ½ĪŖ£ŗCH2=CHCOOH£¬
“š£ŗøĆÓŠ»śĪļµÄ½į¹¹¼ņŹ½ĪŖCH2=CHCOOH£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļ·Ö×ÓŹ½”¢½į¹¹¼ņŹ½µÄČ·¶Ø£¬ĢāÄæÄѶČÖŠµČ£¬×¢Ņā“ÓÖŹĮæŹŲŗćµÄ½Ē¶Č¼ĘĖć£¬Ć÷Č·³£¼ūÓŠ»śĪļ½į¹¹ÓėŠŌÖŹĪŖ½ā“š¹Ų¼ü£¬ŹŌĢāÅąŃųĮĖѧɜµÄ»Æѧ¼ĘĖćÄÜĮ¦£®


 ÖŠæ¼Ąū½£ÖŠæ¼ŹŌ¾ķ»ć±ąĻµĮŠ“š°ø
ÖŠæ¼Ąū½£ÖŠæ¼ŹŌ¾ķ»ć±ąĻµĮŠ“š°ø ½ĢÓżŹĄ¼ŅדŌŖ¾ķĻµĮŠ“š°ø
½ĢÓżŹĄ¼ŅדŌŖ¾ķĻµĮŠ“š°ø »ĘøŌæĪĢĆ×÷Ņµ±¾ĻµĮŠ“š°ø
»ĘøŌæĪĢĆ×÷Ņµ±¾ĻµĮŠ“š°ø µ„ŌŖ¼ÓĘŚÄ©ø“Ļ°ĻČ·ę“óæ¼¾ķĻµĮŠ“š°ø
µ„ŌŖ¼ÓĘŚÄ©ø“Ļ°ĻČ·ę“óæ¼¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
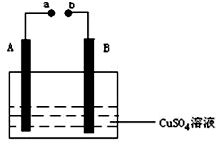 ČēĶ¼ĖłŹ¾×°ÖĆĪŖŌŚÖ±Į÷µēµÄ×÷ÓĆĻĀµē½āCuSO4ČÜŅŗĶ¼£¬ĘäÖŠA”¢BĪŖŹÆÄ«µē¼«£¬a”¢bĪŖµēŌ“µÄĮ½¼«£¬µ±½ÓĶصēŌ“ŗó£¬ĶصēŅ»¶ĪŹ±¼äŗ󣬽«Bµē¼«Č”³öĻ“øɾ»²¢øÉŌļŗó³ĘĮæĘäÖŹĮæŌö¼ÓĮĖ3.2g£¬Ōņ£ŗ
ČēĶ¼ĖłŹ¾×°ÖĆĪŖŌŚÖ±Į÷µēµÄ×÷ÓĆĻĀµē½āCuSO4ČÜŅŗĶ¼£¬ĘäÖŠA”¢BĪŖŹÆÄ«µē¼«£¬a”¢bĪŖµēŌ“µÄĮ½¼«£¬µ±½ÓĶصēŌ“ŗó£¬ĶصēŅ»¶ĪŹ±¼äŗ󣬽«Bµē¼«Č”³öĻ“øɾ»²¢øÉŌļŗó³ĘĮæĘäÖŹĮæŌö¼ÓĮĖ3.2g£¬Ōņ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na+”¢H+”¢Cl-”¢SO42- | B£® | Na+”¢H+”¢Cl-”¢HCO3- | ||
| C£® | Al3+”¢K+”¢OH-”¢NO3- | D£® | NH4+”¢OH-”¢NO3-”¢Na+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗģ×ŲÉ«µÄNO2¼ÓŃ¹ŗóŃÕÉ«ĻȱäÉīŗó±äĒ³ | |
| B£® | øÖĢśŌŚ³±ŹŖµÄæÕĘųÖŠČŻŅ×ÉśŠā | |
| C£® | ĪĀ¶Č¹żø߶ŌŗĻ³É°±²»Ąū | |
| D£® | ³£ĪĀĻĀ£¬½«1mLpH=3µÄ“×ĖįČÜŅŗ¼ÓĖ®Ļ”ŹĶÖĮl00mL£¬²āµĆĘäpH£¼5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ij½šŹōŌŖĖŲĘųĢ¬»łĢ¬Ō×ÓµÄÖš¼¶µēĄėÄܵďżÖµ·Ö±šĪŖ738”¢1451”¢7733”¢10540”¢13630”¢17995”¢21703”£¬µ±ĖüÓėĀČĘų·“Ó¦Ź±Éś³ÉµÄŃōĄė×ÓŹĒX3+ | |
| B£® | “Ö¹č$”ś_{øßĪĀ}^{Cl_{2}}$SiCl4$”ś_{øßĪĀ}^{H_{2}}$Si¾łÄÜŅ»²½ŹµĻÖ | |
| C£® | 33gCH”ŌC-CH=CH-CH3ÖŠĖłŗ¬µÄ¦Š¼üŹż”¢12gŹÆÄ«ÖŠĖłŗ¬µÄĢ¼Ģ¼¼üŹż¾łĪŖ1.5mol | |
| D£® | ×ĘÉÕ°×É«·ŪÄ©£¬»šŃę³É»ĘÉ«£¬Ö¤Ć÷Ō·ŪÄ©ÖŠÓŠNa+£¬æÉÄÜŗ¬ÓŠK+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CO£Øg£© ÓėNa2O2£Øs£©·“Ó¦·Å³ö509kJČČĮæŹ±£¬µē×Ó×ŖŅĘŹżĪŖ6.02”Į1023 | |
| B£® | 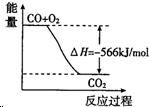 Ķ¼æɱķŹ¾ÓÉCOÉś³ÉCO2µÄ·“Ó¦¹ż³ĢŗĶÄÜĮæ¹ŲĻµ | |
| C£® | 2Na2O2£Øs£©+2CO2£Øs£©ØT2Na2CO3£Øs£©+O2£Øg£©”÷H£¾-452kJ/mol | |
| D£® | COµÄČ¼ÉÕČČĪŖ283kJ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH4+ Fe3+ SO42- NO3- | B£® | K+ Na+ CO32- NO3- | ||
| C£® | K+ NH4+ OH- SO42- | D£® | Na+ K+ AlO2- Cl- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | H+H”śH-H | B£® | H-C1”śH+C1 | ||
| C£® | Mg+2HClØTMgCl2+H2”ü | D£® | H2SO4+2NaOHØTNa2SO4+2H2O |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com