
| NaOHΤπ ΦΕΝ ΐ | NaOH÷’ΒψΕΝ ΐ | |
| ΒΎ“Μ¥Έ | 0.10mL | 18.60mL |
| ΒΎΕΰ¥Έ | 0.30mL | 18.00mL |
Ζ÷Έω Θ®1Θ©œ»ΦΤΥψ≥ωœϊΚΡ«β―θΜ·ΡΤ»ή“ΚΒΡΤΫΨυΧεΜΐΘ§»ΜΚσΗυΨίcΘ®¥ΐ≤βΘ©=$\frac{cΘ®±ξΉΦΘ©ΓΝVΘ®±ξΉΦΘ©}{VΘ®¥ΐ≤βΘ©}$ΦΤΥψ≥ω―ΈΥαΒΡΈο÷ ΒΡΝΩΡ―Ε»ΘΜ
Θ®2Θ©ΗυΨίΒΈΕ®÷’Βψ«Α»ή“ΚΈΣΈό…ΪΘ§ΒΈΕ®÷’Βψ ±»ή“ΚΈΣΚλ…Ϊ≈–ΕœΒΈΕ®÷’ΒψΘΜ
Θ®3Θ©ΗυΨί≤ΌΉςΕ‘cΘ®¥ΐ≤βΘ©=$\frac{cΘ®±ξΉΦΘ©ΓΝVΘ®±ξΉΦΘ©}{VΘ®¥ΐ≤βΘ©}$ΒΡ”ΑœλΫχ––Ζ÷ΈωΒΈΕ®Έσ≤νΘ°
Ϋβ¥π ΫβΘΚΘ®1Θ©ΗυΨί±μΗώ÷– ΐΨίΩ…÷ΣΘ§ΒΎ“Μ¥ΈΒΈΕ®œϊΚΡ«β―θΜ·ΡΤ»ή“ΚΒΡΧεΜΐΈΣΘΚΘ®18.60-0.10Θ©mL=18.50mLΘ§ΒΎΕΰ¥ΈΒΈΕ®œϊΚΡ«β―θΜ·ΡΤΒΡΧεΜΐΈΣΘΚΘ®18.00-0.30Θ©mL=17.70mLΘ§œϊΚΡ«β―θΜ·ΡΤΧεΜΐΒΡΤΫΨυ÷ΒΈΣΘΚ$\frac{18.50+17.70}{2}$mL=18.10 mLΘ§
cΘ®NaOHΘ©=$\frac{0.2ΓΝ0.0181}{0.025}$mol/LΓ÷0.1448 mol/LΘ§
Ι ¥πΑΗΈΣΘΚ0.1448ΘΜ
Θ®2Θ©ΒΈΕ®Ϋα χ÷°«ΑΘ§»ή“ΚΈΣΈό…ΪΘ§ΒΈΕ®Ϋα χ ±»ή“Κ±δ≥…Κλ…ΪΘ§Υυ“‘ΒΈΕ®÷’Βψœ÷œσΈΣΘΚΒΈΉνΚσ“ΜΒΈ»ή“Κ”…Έό…Ϊ±δ«≥Κλ…ΪΘ§ΑκΖ÷÷”≤ΜΆ …ΪΘ§
Ι ¥πΑΗΈΣΘΚΒΈΉνΚσ“ΜΒΈ»ή“Κ”…Έό…Ϊ±δ«≥Κλ…ΪΘ§ΑκΖ÷÷”≤ΜΆ …ΪΘΜ
Θ®3Θ©AΘ°≈δ÷Τ±ξΉΦ»ή“ΚΒΡ«β―θΜ·ΡΤ÷–Μλ”–Na2CO3‘”÷ Θ§ΒΦ÷¬VΘ®NaOHΘ©ΤΪΗΏΘ§ΨίcΘ®¥ΐ≤βΘ©=$\frac{cΘ®±ξΉΦΘ©ΓΝVΘ®±ξΉΦΘ©}{VΘ®¥ΐ≤βΘ©}$Ζ÷ΈωΘ§cΘ®¥ΐ≤βΘ©ΤΪΗΏΘ§Ι A’ΐ»ΖΘΜ
BΘ°ΒΈΕ®÷’ΒψΕΝ ΐ ±Θ§Η© ”ΒΈΕ®ΙήΒΡΩΧΕ»Θ§ΤδΥϋ≤ΌΉςΨυ’ΐ»ΖΘ§ΒΦ÷¬VΘ®NaOHΘ©ΤΪΒΆΘ§ΨίcΘ®¥ΐ≤βΘ©=$\frac{cΘ®±ξΉΦΘ©ΓΝVΘ®±ξΉΦΘ©}{VΘ®¥ΐ≤βΘ©}$Ζ÷ΈωΘ§cΘ®¥ΐ≤βΘ©ΤΪΒΆΘ§Ι B¥μΈσΘΜ
CΘ° ΔΉΑΈ¥÷Σ“ΚΒΡΉΕ–ΈΤΩ”Ο’τΝσΥ°œ¥ΙΐΘ§Έ¥”Ο¥ΐ≤β“Κ»σœ¥Θ§≤ΌΉς’ΐ»ΖΘ§Ε‘ΫαΙϊΈό”ΑœλΘ§Ι C¥μΈσΘΜ
DΘ°ΒΈΕ®ΒΫ÷’ΒψΕΝ ΐ ±ΖΔœ÷ΒΈΕ®ΙήΦβΉλ¥Π–ϋΙ““ΜΒΈ»ή“ΚΘ§ΒΦ÷¬VΘ®NaOHΘ©ΤΪΗΏΘ§ΨίcΘ®¥ΐ≤βΘ©=$\frac{cΘ®±ξΉΦΘ©ΓΝVΘ®±ξΉΦΘ©}{VΘ®¥ΐ≤βΘ©}$Ζ÷ΈωΘ§cΘ®¥ΐ≤βΘ©ΤΪΗΏΘ§Ι D’ΐ»ΖΘΜ
Ι ―ΓADΘ°
ΒψΤά ±ΨΧβ÷ς“ΣΩΦ≤ιΝΥΥαΦν÷–ΚΆΒΈΕ®ΦΑΈσ≤νΖ÷ΈωΘ§ΧβΡΩΡ―Ε»÷–Β»Θ§ΉΔ“βΥαΦν÷–ΚΆΒΈΕ®ΒΡ≤ΌΉςΖΫΖ®ΦΑΈσ≤νΖ÷ΈωΒΡΖΫΖ®Θ§ ‘Χβ≈ύ―χΝΥ―ß…ζΒΡΖ÷ΈωΓΔάμΫβΡήΝΠΦΑΝιΜν”Π”ΟΥυ―ß÷Σ ΕΒΡΡήΝΠΘ§ «“ΜΒά≤Μ¥μΒΡ ‘ΧβΘ°



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | ÷ΜΚ§1ΗωΧΦΧΦΥΪΦϋΒΡ÷±Ν¥”–ΜζΈο | BΘ° | Κ§2ΗωΧΦΧΦΥΪΦϋΒΡ÷±Ν¥”–ΜζΈο | ||
| CΘ° | Κ§1ΗωΧΦΧΦΥΪΦϋΒΡΜΖΉ¥”–ΜζΈο | DΘ° | Κ§1ΗωΧΦΧΦ»ΐΦϋΒΡ÷±Ν¥”–ΜζΈο |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | ’τΖΔ | BΘ° | ÷ΊΫαΨß | CΘ° | ’τΝσ | DΘ° | Ζ÷“Κ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ

| AΘ° | ΔΌΔή | BΘ° | ΔΌΔΎΔή | CΘ° | ΔΎΔέΔή | DΘ° | ΔΎΔέ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | NH${\;}_{4}^{+}$ΓΔH+ΓΔNO${\;}_{3}^{-}$ΓΔHCO${\;}_{3}^{-}$ | BΘ° | K+ΓΔAl3+ΓΔSO${\;}_{4}^{2-}$ΓΔNH3•H2O | ||
| CΘ° | Na+ΓΔK+ΓΔSO${\;}_{3}^{2-}$ΓΔCl2 | DΘ° | Na+ΓΔCl-ΓΔCO${\;}_{3}^{2-}$ΓΔOH- |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | Έό…Ϊ»ή“Κ÷–ΘΚK+ΓΔCu2+ΓΔNa+ΓΔSO42- | |
| BΘ° | ‘Ύ”…Υ°Βγάκ≥ωΒΡcΘ®OH-Θ©=10-13 mol•L-1ΒΡ»ή“Κ÷–ΘΚNa+ΓΔBa2+ΓΔCl-ΓΔI- | |
| CΘ° | Ρή ΙΚλ…Ϊ ·»ο ‘÷Ϋ±δΈΣάΕ…ΪΒΡ»ή“ΚΘΚNa+ΓΔCl-ΓΔS2-ΓΔClO- | |
| DΘ° | Κ§”–¥σΝΩClO-ΒΡ»ή“ΚΘΚH+ΓΔI-ΓΔSO42-ΓΔCl- |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | »ή“Κ÷–Κ§”–H+ | BΘ° | ΒΈ»κΖ”ΧΣ ‘“ΚΚσΘ§»ή“Κ≥ Κλ…Ϊ | ||
| CΘ° | cΘ®OH-Θ©ΘΨcΘ®H+Θ©ΒΡ»ή“Κ | DΘ° | ”κΫπ τΧζΖ¥”ΠΖ≈≥ω«βΤχ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ―Γ‘ώΧβ
| AΘ° | S‘≠Ή”ΒΡΫαΙΙ Ψ“βΆΦΘΚ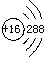 | |
| BΘ° | ¬»Μ·ΡΤΒΡΒγΉ” ΫΘΚ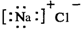 | |
| CΘ° | ““¥ΦΒΡΫαΙΙ ΫΘΚ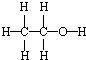 | |
| DΘ° | ΝρΥαΒΡΒγάκΖΫ≥Χ ΫΘΚHSO4=H2++SO42- |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΫβ¥πΧβ
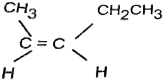
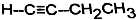
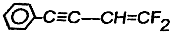
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com