
 £®
£®·ÖĪö £Ø1£©0.2molCxHy”ś1.2molCO2+1.2molH2O£¬ÓÉŌ×ÓŹŲŗć·ÖĪö£»
£Ø2£©A²»ÄÜŹ¹äåĖ®ĶŹÉ«£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀÄÜÓėĀČĘų·¢ÉśČ”“ś·“Ó¦£¬ĘäŅ»ĀČ“śĪļÖ»ÓŠŅ»ÖÖ£¬ŌņÖ»ÓŠŅ»ÖÖH£¬ĪŖ»·¼ŗĶ飻
£Ø3£©ČōĢžAÄÜŹ¹äåĖ®ĶŹÉ«£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀÓėH2¼Ó³ÉÉś³É£¬Ęä¼Ó³É²śĪļ¾²ā¶Ø£¬·Ö×ÓÖŠŗ¬ÓŠ4øö¼×»ł£¬
Éś³ÉµÄĶéĢž½į¹¹Ź½ĪŖ »ņ
»ņ £¬Ęä¼Ó³É²śĪļB¾²ā¶Ø·Ö×ÓÖŠŗ¬ÓŠ4øö¼×»ł£¬ĒŅĘäŗĖ“Ź²ÕńĒāĘ×Ķ¼ÖŠÓŠĮ½×é·å£¬Ó¦ĪŖ
£¬Ęä¼Ó³É²śĪļB¾²ā¶Ø·Ö×ÓÖŠŗ¬ÓŠ4øö¼×»ł£¬ĒŅĘäŗĖ“Ź²ÕńĒāĘ×Ķ¼ÖŠÓŠĮ½×é·å£¬Ó¦ĪŖ £¬ŅŌ“ĖČ·¶ØAµÄ½į¹¹¼ņŹ½£®
£¬ŅŌ“ĖČ·¶ØAµÄ½į¹¹¼ņŹ½£®
½ā“š ½ā£ŗ£Ø1£©0.2molĢžAŌŚŃõĘųÖŠĶźČ«Č¼ÉÕŗó£¬Éś³ÉCO2ŗĶH2Oø÷1.2mol£¬ŌņA·Ö×ÓÖŠŗ¬ÓŠC”¢HŌ×ÓŹż·Ö±šĪŖ£ŗN£ØC£©=$\frac{1.2mol}{0.2mol}$=6£¬n£ØH£©=$\frac{1.2mol”Į2}{0.2mol}$=12£¬A·Ö×ÓŹ½ĪŖ£ŗC6H12£¬
¹Ź“š°øĪŖ£ŗC6H12£»
£Ø2£©C6H12Ö»ÓŠ1øö²»±„ŗĶ¶Č£¬ČōĢžA²»ÄÜŹ¹äåĖ®ĶŹÉ«£¬ŌņĘäĪŖ»·ĶéĢž£®ĘäÖŠÄÜÓėĀČĘų·¢ÉśČ”“ś·“Ó¦£¬ĘäŅ»ĀČ“śĪļÖ»ÓŠŅ»ÖÖ£¬ĖµĆ÷Ęä·Ö×ÓÖŠÖ»ÓŠ1ÖֵȊ§H£¬¹ŹøĆÓŠ»śĪļŹĒ»·¼ŗĶ飬½į¹¹¼ņŹ½ĪŖ£ŗ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø3£©ĢžAÄÜŹ¹äåĖ®ĶŹÉ«£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀ£¬ÓėH2¼Ó³É£¬Ęä¼Ó³É²śĪļ¾²ā¶Ø·Ö×ÓÖŠŗ¬ÓŠ4øö¼×»ł£¬ĖµĆ÷ĢžÖŠŗ¬ÓŠC=C£¬ĘäÖŠŗ¬ÓŠ4øö¼×»łµÄÓŠ3ÖÖ£¬ĘäĢ¼¼Ü½į¹¹ĪŖ£Ø¢Ł¢Ś¢Ū“¦æÉ·Ö±š²»Ķ¬Ź±°²ÅÅĖ«¼ü£© £¬æÉÄÜÓŠµÄ½į¹¹¼ņŹ½ĪŖ£ŗ£ØCH3£©3C-CH=CH2”¢CH3-C£ØCH3£©=C£ØCH3£©-CH3”¢CH3CH£ØCH3£©-C£ØCH3£©=CH2£»ÓÖB·Ö×ÓÖŠŗĖ“Ź²ÕńĒāĘ×Ķ¼ÖŠÓŠĮ½×é·å£¬ĖµĆ÷B·Ö×Ó¾ßÓŠ¶Ō³Ę½į¹¹£¬Āś×ćĢõ¼žµÄB½į¹¹¼ņŹ½ĪŖ£ŗCH3-CH£ØCH3£©CH£ØCH3£©-CH3£¬ĘäĆū³ĘĪŖ£ŗ2£¬3-¶ž¼×»ł¶”Ķ飬ŌņAµÄ½į¹¹¼ņŹ½ĪŖ£ŗCH3-C£ØCH3£©=C£ØCH3£©-CH3£¬
£¬æÉÄÜÓŠµÄ½į¹¹¼ņŹ½ĪŖ£ŗ£ØCH3£©3C-CH=CH2”¢CH3-C£ØCH3£©=C£ØCH3£©-CH3”¢CH3CH£ØCH3£©-C£ØCH3£©=CH2£»ÓÖB·Ö×ÓÖŠŗĖ“Ź²ÕńĒāĘ×Ķ¼ÖŠÓŠĮ½×é·å£¬ĖµĆ÷B·Ö×Ó¾ßÓŠ¶Ō³Ę½į¹¹£¬Āś×ćĢõ¼žµÄB½į¹¹¼ņŹ½ĪŖ£ŗCH3-CH£ØCH3£©CH£ØCH3£©-CH3£¬ĘäĆū³ĘĪŖ£ŗ2£¬3-¶ž¼×»ł¶”Ķ飬ŌņAµÄ½į¹¹¼ņŹ½ĪŖ£ŗCH3-C£ØCH3£©=C£ØCH3£©-CH3£¬
¹Ź“š°øĪŖ£ŗCH3-C£ØCH3£©=C£ØCH3£©-CH3£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄĶʶĻ£¬ĪŖøßĘµæ¼µć£¬ĢāÄæÄѶČÖŠµČ£¬ŹģĮ·ÕĘĪÕ³£¼ūÓŠ»śĪļ½į¹¹ÓėŠŌÖŹĪŖ½ā“š¹Ų¼ü£¬ŹŌĢā²ąÖŲÓŚĶ¬·ÖŅģ¹¹ĢåµÄÅŠ¶Ļ£¬×¢Ņāøł¾ŻĪļÖŹµÄŠŌÖŹÅŠ¶ĻæÉÄܾßÓŠµÄ½į¹¹£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| ŹµŃé“ĪŹż | 1 | 2 | 3 |
| ĻūŗÄNa2S2O3ČÜŅŗĢå»ż/mL | 19.30 | 20.98 | 21.02 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | X”¢YµÄÅØ¶Č²»ŌŁ±ä»Æ | |
| B£® | µ„Ī»Ź±¼äÉś³ÉamolX£¬Ķ¬Ź±Éś³É3amolY | |
| C£® | X”¢Y”¢ZµÄ·Ö×ÓŹżÖ®±ČĪŖ1£ŗ3£ŗ2 | |
| D£® | XÕ¼»ģŗĻĘųĢåµÄĢå»ż·ÖŹż²»±ä |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĖłµĆČÜŅŗµÄĢå»żĪŖ22.5L | |
| B£® | øĆČÜŅŗĪļÖŹµÄĮæÅضČĪŖ10.00mol•L-1 | |
| C£® | øł¾ŻĢāÖŠŹż¾Ż£¬ĪŽ·ØĒóµĆøĆČÜŅŗĪļÖŹµÄĮæÅØ¶Č | |
| D£® | øĆČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżŅņČÜŅŗµÄĆܶČĪ“ÖŖ¶ųĪŽ·ØĒóµĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | C2H4ŗĶC4H8 | B£® | ÓĶĖįŗĶ±ūĻ©Ėį | ||
| C£® |  ŗĶ ŗĶ | D£® | ÕįĢĒŗĶĀóŃæĢĒ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĖÜĮĻ | B£® | µķ·Ū | C£® | ÓĶÖ¬ | D£® | µ°°×ÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | £Ø1£©£Ø2 £© £Ø3£© | B£® | £Ø2£©£Ø3£©£Ø4£© | C£® | £Ø2£©£Ø4£©£Ø5£© | D£® | £Ø1£©£Ø3£©£Ø5£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
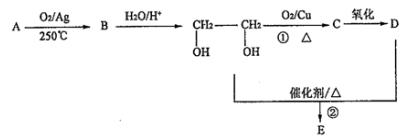
 £®
£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com