
2SO2(g)��O2(g)��2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1 mol��SO2(g)����Ϊ1 mol��SO3����H����99 kJ��mol��1����ش��������⣺

(1)ͼ��A��C�ֱ��ʾ________��________��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿(���Ӱ�족����Ӱ�족)________���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ�________��������________��
(2)ͼ�С�H��________KJ��mol��1��
(3)V2O5�Ĵ�ѭ����������Ϊ��V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�����������д���ô�ѭ�������Ļ�ѧ����ʽ________��
(4)�����Ӧ������(SO2)Ϊ0.05 mol��L��1��min��1������(O2)��________mol��L��1��min��1����(SO3)��________mol��L��1��min��1��
(5)��֪�������ȼ����Ϊ296 KJ��mol��1��������S(s)����3 mol��SO3(g)�ġ�H________(Ҫ��������)��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���淴Ӧ2SO2(g)��O2(g)2SO3(g)����H<0��һ�������´ﵽƽ��״̬��ʱ��Ϊt1ʱ�ı���������ѧ��Ӧ�����뷴Ӧʱ���ϵ��ͼ������˵������ȷ����
A��ά���¶ȡ���Ӧ��ϵ������䣬t1ʱ����SO3(g)
B��ά��ѹǿ���䣬t1ʱ���߷�Ӧ��ϵ�¶�
C��ά���¶Ȳ��䣬t1ʱ����Ӧ��ϵ���
D��ά���¶ȡ�ѹǿ���䣬t1ʱ����SO3(g)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ��������������ѧ�߶�5���¿���ѧ�Ծ����������� ���ͣ���ѡ��
���ڷ�Ӧ2SO2(g)��O2(g) 2SO3(g)������������Ӧ���ʵĴ�ʩ��
2SO3(g)������������Ӧ���ʵĴ�ʩ��
| A��ͨ�����O2 | B�����������ݻ� | C����ȥ����SO3 | D��������ϵ�¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�캣��ʡ�����и߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ������
2SO2(g)��O2(g) 2SO3(g)����Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 mol SO3(g)�Ħ�H����99 kJ/mol��
2SO3(g)����Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 mol SO3(g)�Ħ�H����99 kJ/mol��
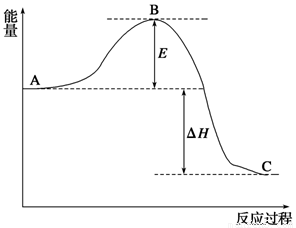
��ش��������⣺
��1��ͼ��A��C�ֱ��ʾ__________��__________��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿____________���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ�___________��
��2��ͼ�Ц�H��__________kJ/mol��
��3�������Ӧ����v(SO2)Ϊ0.05 mol/(L��min)����v(O2)��____mol/(L��min)
��4����֪�������ȼ����Ϊ296 kJ/mol��������S(s)����3 mol SO3(g)�Ħ�H��_ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�켪��ʡ�����и�����ѧ�����Ի�ѧ�Ծ��������棩 ���ͣ������
SO2��NOx�ڻ�ѧ��ҵ������Ҫ��;��Ҳ�Ǵ�����Ⱦ����Ҫ��Դ�����������ò��أ�Ԥ�������������ǵ�ǰ��ҵ�Ϻͻ������������о�����Ҫ����֮һ��
��1���ڽӴ���������Ĺ����У�����2SO2(g)��O2(g)
 2SO3(g)
��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
2SO3(g)
��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
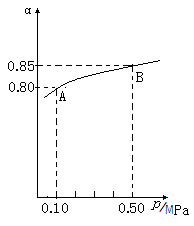
��ƽ��״̬��A��Bʱ��ƽ�ⳣ��K(A) K(B)�����������������������
�ڽ�2��0molSO2��1��0molO2����10L���ܱ������У���40s��Ӧ�ﵽƽ�⣬��ʱ��ϵ��ѹǿΪ0��10MPa����һ��ʱ����SO2��ƽ����Ӧ����Ϊ ��
�÷�Ӧ��ƽ�ⳣ��Ϊ ��
��2����CH4����ԭNOx�������������������Ⱦ�����磺
CH4(g)��4NO2(g) �� 4NO(g)��CO2(g)��2H2O(g) ��H����574kJ��mol��1
CH4(g)��4NO(g) �� 2N2(g)��CO2(g)��2H2O(g) ��H����1160kJ��mol��1
ȡ��״����4��48LCH4��ʹ֮��ȫ��Ӧ��
������NO2��ԭ��N2������������ת�Ƶ��ӵ����ʵ���Ϊ ��
������ԭNO2��NO�Ļ����ų���������Q��ȡֵ��Χ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡפ������ȷɽ���߸߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ѡ����
��֪��Ӧ��2SO2(g)��O2(g)
 2SO3(g)����H<0��ij�¶��£���2 mol SO2��1 mol O2����10 L�ܱ������У���Ӧ��ƽ���SO2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ����
2SO3(g)����H<0��ij�¶��£���2 mol SO2��1 mol O2����10 L�ܱ������У���Ӧ��ƽ���SO2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ����
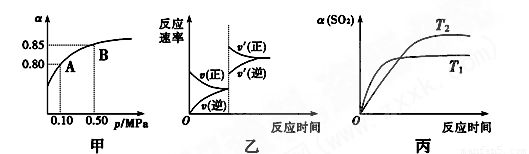
A����ͼ��֪��A��SO2��ƽ��Ũ��Ϊ0.4 mol��L��1
B����ͼ��֪��B��SO2��O2��SO3��ƽ��Ũ��֮��Ϊ2��1��2
C����ƽ�����С�����ݻ�����Ӧ���ʱ仯ͼ�������ͼ�ұ�ʾ
D��ѹǿΪ0.50 MPaʱ��ͬ�¶���SO2ת�������¶ȹ�ϵ���ͼ����T2>T1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com