
����Ŀ��ij����ˮ�к�5.00��10-3mol��L-1��![]() ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���![]() ��
��![]() �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
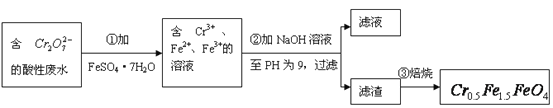
��1����������Ӧ�����ӷ���ʽ��_________________________________________________��
��2������������pH��ֽ�ⶨ��ҺpH�IJ����ǣ�
______________________________________________________________________________��
��3�����������˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����______________________��
��4����ʹ1L�÷�ˮ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����������Ҫ����__________g FeSO4��7H2O��
����������Ҫ����__________g FeSO4��7H2O��
���𰸡�Cr2O72-+ 6Fe2��+ 14H��![]() 2Cr3+ + 6Fe3��+ 7H2O ��һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ������ Fe(OH)3��Fe(OH)2 13.9
2Cr3+ + 6Fe3��+ 7H2O ��һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ������ Fe(OH)3��Fe(OH)2 13.9
��������
(1)Cr2O72-�н�ǿ�����ԣ�FeSO47H2O��Fe2+��һ���Ļ�ԭ�ԣ������Խ����з���������ԭ��Ӧ����ʵ�����̿�֪���ڢٲ���Ӧ��Cr2O72-�����������½�Fe2+����ΪFe3+����������ԭΪCr3+�������غ�Ԫ���غ㼰����������֪����Ӧ��ˮ���ɣ���Ӧ���ӷ���ʽΪCr2O72-+14H++6Fe2+=2Cr3++6Fe3++7H2O���ʴ�Ϊ��Cr2O72-+14H++6Fe2+=2Cr3++6Fe3++7H2O��
(2)��һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��ʴ�Ϊ����һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ�����գ�
(3)�������ͼ�ɵã�Fe2+��������NaOHʱ������Cr(OH)3��Fe(OH)3��Fe(OH)2���ֳ�����ʴ�Ϊ��Fe(OH)3��Fe(OH)2��
(4)1 L��ˮ�к�n(Cr2O72-)=5.00��10-3 mol������Crԭ�ӡ�Feԭ���غ㣬�ɵã�Cr2O72-������4Cr0.5Fe1.5FeO4������10FeSO47H2O������������n(FeSO47H2O)=10n(Cr2O72-)=5.00��10-3 mol��10=0.05 mol������m(FeSO47H2O)=0.05 mol��278 g/mol=13.9 g���ʴ�Ϊ��13.9g��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش���������
(1)������Ϊ17��������Ϊ20��ԭ�ӿɱ�ʾΪ________��Na+�Ľṹʾ��ͼ________��
(2)��ɫ��Ӧ��_______�仯����̼���ƽ�����ɫ��Ӧ�������________��
(3)Ư����Ч�ɷֵĻ�ѧʽ________��Ư�۷���Ư�����õķ�Ӧ����ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ�������
A. 1L0.1mol/L��NH4Cl��Һ�У�NH4+����ĿΪ0.1NA
B. 2.4gMg��H2SO4��ȫ��Ӧ��ת�Ƶĵ�����Ϊ0.1NA
C. 0.1molH2��0.1 mol I2���ܱ������г�ַ�Ӧ�����������Ϊ0.2NA
D. ��״���£�11.2LCH2Cl2�к���������Ϊ21.5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ����A�г���1molX��1molY����B�г���2molX��2molY����ʼVA��VB��aL������ͬ�¶Ⱥ��д����������£��������и��Է������з�Ӧ X(g)+Y(g)![]() 2Z(g)+W(g)�ﵽƽ��ʱ��VA��1.2aL��������˵����ȷ���ǣ�������
2Z(g)+W(g)�ﵽƽ��ʱ��VA��1.2aL��������˵����ȷ���ǣ�������

A. ��Ӧ��ʼʱ��B�����л�ѧ��Ӧ���ʿ�
B. A������X��ת����Ϊ40%���ұ�B������X��ת����С
C. ��Kһ��ʱ���ƽ��ʱ��A�����Ϊ1.6aL(��ͨ���������������)
D. ��K����ƽ�������B�����¶ȣ�A�������һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�к�����Ϊ���뷴Ӧ������ʵ�����������Ϊ�������������ʵ���������ѡ��ı�ź����ߵı��һһ��Ӧ�����������������
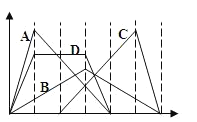
A. ��NaAlO2��Һ������HCl������
B. �����ʯ��ˮ��ͨ��CO2������
C. ���������AlCl3��Һ�е���NaOH��Һ������
D. ���е����ʵ�����Ca(OH)2��KOH�Ļ����Һ��ͨ��CO2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����⻯��(NaBH4����Ϊ+3��)Ϊ��ɫ��ĩ���ڸ���������ȶ����ڳ�ʪ�����зֽ⣬�dz��õĻ�ԭ����ƫ�����ƣ�NaBO2��������ˮ���������Ҵ�����ˮ�⡣Ŀǰ�ж��ֹ��տ��Ʊ�NaBH4��
��1��������һ����B2O3����Al2O3��SiO2��Fe2O3�����ʣ���ȡNaBH4���������£�
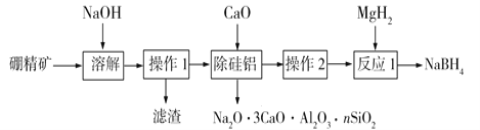
�١��ܽ���ʱ��B2O3��NaOH��Ӧ������NaBO2����Ӧ���ӷ���ʽΪ____��
�ڡ����������������CaO����������CaCl2��ԭ���У��ܽ��衢���Գ�����ȥ�������������������ӣ�___��
�ۡ�����2���ǽ���Һ�������ᾧ��ϴ�ӣ�����ϴ��ѡ�õ��Լ������_____������ĸ����
a. ��ˮ���� b. �Ҵ��� c. ��ˮ���� d. NaOH��Һ
������Ӧ1����MgH2��NaBO2��ϵõ�NaBH4��MgO���仯ѧ����ʽΪ________��
��2���ҹ�����ƽ������ʴ�缫���ϣ��������ӽ���ĤΪ����Ĥ�����ƫ�����Ƶļ���Һ��Ҳ���Ը�Ч�Ʊ�NaBH4���ù�����������Ϊ________�������缫����ʽΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ǹ�ˮ����[Cr(CH3COO)2]22H2O������Ϣ���£�
�������� | ��ѧ���� |
����ɫ���壬�����Ҵ�����������ˮ������(�ӷ����л��ܼ�) | ����ǿ��ԭ�ԣ��ױ����� |
�Ʊ�ԭ����2Cr2��(aq)��4CH3COO��(aq)��2H2O(l)��[Cr(CH3COO)2]22H2O(s)��
ij��ȤС�����ʵ���Ʊ�[Cr(CH3COO)2]22H2O(s)��

�ش��������⣺
(1)����A��������_____��
(2)���װ��B�����ԵIJ���������_____��
(3)��������ر�K1����K2����װ��B�еĵ��ܳ��Һ������һ��ʱ�䣬Ŀ����____����Ӧ��ʼ��װ��B�п�������������Һ������ɫ(Cr3��)��Ϊ����ɫ(Cr2��)���������ݲ�����д��װ��B�з����ķ��û���Ӧ�����ӷ���ʽ��_____��
(4)�������ķų����ʽϿ�ʱ��Ϊ��ʹװ��B����Һ����װ��C�У��˲�ȡ�IJ�����_____��װ��D�е��ܿ�ˮ���Ŀ����_____��
(5)��װ��C�����ò�Ʒ�ᴿ�����������Ϊ���ˡ�ȥ��ˮϴ�ӡ�����ϴ�ӡ�����Ҵ���ˮ��������ϴ�ӵ��ŵ���______��
(6)�ⶨ��Ʒ���ȣ�ȡag��Ʒ��������ˮ��ͨ��������������ַ�Ӧ�����������������Һ�����ˡ�ϴ�ӡ����ա����ء���Cr2O3����Ϊmg(�������ʲ����뷴Ӧ)��[Cr(CH3COO)2]22H2O(s)��Ħ������ΪMg��mol��1����ò�Ʒ����Ϊ____ %��(�ú�a��m��M�Ĵ���ʽ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵�����ʾ������ȷ���ǣ� ��
A��Cȼ�յ��Ȼ�ѧ����ʽΪ��C(s)��1/2O2(g)=CO(g) ��H = 110.5kJ��mol-1
B����H+��aq��+OH-��aq��=H2O��l�� ��H=-57.3kJ��mol-1��֪��������1molCH3COOH��ϡ��Һ�뺬1molNaOH��ϡ��Һ��ϣ��ų�������С��57.3kJ
C��̼������ˮ������ӷ���ʽ��HCO3����H2O![]() CO32����H3O+
CO32����H3O+
D��500����30MPa�£���0.5molN2��g����1.5mol H2��g�������ܱ������г�ַ�Ӧ����NH3��g�����ų�����19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��![]() 2NH3��g�� ��H=-38.6kJ��mol-1
2NH3��g�� ��H=-38.6kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���
��1��д����������NaOHˮ��Һ���ȵķ�Ӧ����ʽ��_______________��ijͬѧȡ������������NaOHˮ��Һ��Ӧ��Ļ����Һ�������еμ�AgNO3��Һ�����ȣ�����������������ͬѧ�ɴ˵ó���������NaOHˮ��Һ��Ӧ���������廯�ƣ�����Ϊ�Ƿ������ԭ��____________________��
��2��д����������NaOH�Ҵ���Һ���ȵķ�Ӧ����ʽ��________����Ӧ�����ɵ������������ͼ��ʾװ�ü��飬������______��ˮ�������� ______��
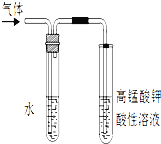
��3����ϩʹ��ˮ��ɫ�Ļ�ѧ����ʽ�� __________________________��X�DZ���ϩ��Է���������14����ϩ��ͬϵ���ҵ����X�������ϵĻ�ѧ����ʽΪ��___________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com