
(1)�µġ�����������������(GB 3095 2012)����2016��1��1�����ҹ�ȫ��ʵʩ���ݴ�,������������ָ��(AQI)�ձ���ʵʱ���������SO2��NO2��CO��O3��PM10��PM2.5��ָ��,Ϊ�����ṩ����ָ��,�������ؾ���������ų��к����
2012)����2016��1��1�����ҹ�ȫ��ʵʩ���ݴ�,������������ָ��(AQI)�ձ���ʵʱ���������SO2��NO2��CO��O3��PM10��PM2.5��ָ��,Ϊ�����ṩ����ָ��,�������ؾ���������ų��к����
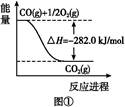
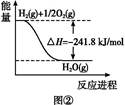
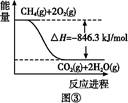
�������ų���β���к���CO��NO������,�û�ѧ����ʽ���Ͳ���NO��ԭ�� ��
�������������ڰ�װ�Ĵ�ת����,��ʹ����β���е���Ҫ��Ⱦ��ת��Ϊ���Ĵ���ѭ�����ʡ���֪:
N2(g)+O2(g)="2NO(g)" ��H="+180.5" kJ/mol
2C(s)+O2(g)="2CO(g)" ��H="-221.0" kJ/mol
C(s)+O2(g)=CO2(g) ��H="-393.5" kJ/mol
��Ӧ2NO(g)+2CO(g)=N2(g)+2CO2(g)�Ħ�H= kJ/mol��
(2)ֱ���ŷŵ���������γ����ꡢ����,����ԭ�����������շ��dz��õĴ�������������NH3��CH4�������ȥ�����еĵ��������֪:CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H1="a" kJ/mol;�����㷴ӦCH4(g)+4NO(g)=CO2(g)+2H2O(l)+2N2(g)���ʱ䦤H2����Ҫ��ѯij��Ӧ���ʱ䦤H3,����Ӧ�и����ʵĻ�ѧ������֮��Ϊ���������ʱ,��H3="b" kJ/mol,�÷�Ӧ���Ȼ�ѧ����ʽ�� ,�ݴ˼������H2= kJ/mol(�ú�a��b��ʽ�ӱ�ʾ)��
(3)�±��г��˹�ҵ������SO2�����ַ�����
| ������ | �ð�ˮ��SO2ת��(NH4)2SO3,��������(NH4)2SO4 |
| ������ | ���������Ƚ���(��Ҫ�ɷ�CO��CH4��H2)��SO2�ڸ����»�ԭ�ɵ����� |
| ������ | ��Na2SO3��Һ����SO2,�پ����ת��ΪH2SO4 |
 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
NOx������β���е���Ҫ��Ⱦ��֮һ��
(1)NOx���γ����꣬д��NO2ת��ΪHNO3�Ļ�ѧ����ʽ��__________________________��
(2)��������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�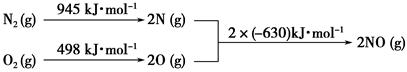
��д���÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
�����¶����ߣ��÷�Ӧ��ѧƽ�ⳣ���ı仯������____��
(3)������β��ϵͳ��װ�ô�ת����������Ч����NOx���ŷš�
�ٵ�β���п�������ʱ��NOx�ڴ�ת�����б���ԭ��N2�ų���д��NO��CO��ԭ�Ļ�ѧ����ʽ��______________________________
�ڵ�β���п�������ʱ����ת�����еĽ�������������NOx�����Ρ�����������˳�����£�12MgO��20CaO��38SrO��56BaO��ԭ����___________________________________________��
Ԫ�صĽ���������ǿ�������������NOx��������������ǿ��
(4)ͨ��NOx�������ɼ��NOx�ĺ������乤��ԭ��ʾ��ͼ���£�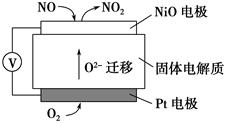
��Pt�缫�Ϸ�������________��Ӧ(���������ԭ��)
��д��NiO�缫�ĵ缫��Ӧʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ѣ�CH3OCH3����һ����Ҫ�ľ�ϸ������Ʒ������Ϊ�Ƕ�ʮһ��������DZ����ȼ��[ ��֪��CH3OCH3(g)+3O2(g)��2CO2(g)+3H2O��1�� ��H����1455kJ/mol ]��ͬʱ��Ҳ������Ϊ�������������ȴ�������ҵ���Ʊ������ѵ���Ҫ���������������Σ�
�ټ״�Һ����Ũ���������»�״������ڴ�������ֱ����ˮ�ƶ����ѣ� 2CH3OH  CH3OCH3��H2O
CH3OCH3��H2O
�ںϳ���CO��H2ֱ�Ӻϳɶ����ѣ� 3H2(g)��3CO(g) CH3OCH3(g)��CO2(g) ��H����247kJ/mol
CH3OCH3(g)��CO2(g) ��H����247kJ/mol
����Ȼ����ˮ������Ӧ�Ʊ������ѡ���CH4��H2OΪԭ���Ʊ������Ѻͼ״���ҵ�������£�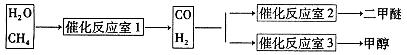
��1��д��CO(g)��H2(g)��O2(g)��Ӧ����CO2(g)��H2O��1�����Ȼ�ѧ����ʽ���������һλС����
��2���ڷ�Ӧ��2�У�һ�������·�����Ӧ3H2(g)��3CO(g) CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��
CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��
A�����¸�ѹ B���Ӵ��� C������COŨ�� D�������������
��3���ڷ�Ӧ��3�У���һ���¶Ⱥ�ѹǿ�����·����˷�Ӧ��3H2(g)��CO2(g)  CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ�
CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ�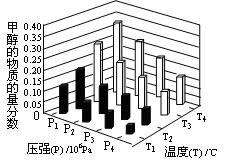
A��P3��P2 T3��T2 B��P2��P4 T4��T2
C��P1��P3 T1��T3 D��P1��P4 T2��T3
��4����Ӧ��1�з�����Ӧ��CH4(g)��H2O(g) CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ��
CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ��
����¶Ƚ��ͣ��÷�Ӧ��ƽ�ⳣ�� ������䡱�����������С����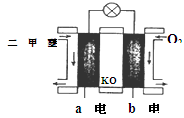
��5����ͼΪ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a�缫�ķ�ӦʽΪ��________________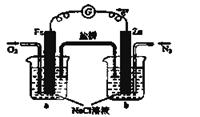
��6�������ж�����ȷ����_______
A�����ձ�a�м�������K3[Fe(CN)6]��Һ������ɫ��������
B���ձ�b�з�����ӦΪ2Zn-4e�� ��2Zn2+
C�����Ӵ�Zn������������Fe���������Żص�Zn��
D���ձ�a�з�����ӦO2 + 4H++ 4e�� �� 2H2O����ҺpH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�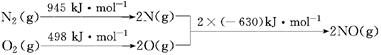
д���÷�Ӧ���Ȼ�ѧ����ʽ��________________��
��2��������(CH3OCH3)����ɫ���壬����Ϊһ��������Դ���ɺϳ���(���ΪH2��CO��������CO2)ֱ���Ʊ������ѣ����е���Ҫ���̰��������ĸ���Ӧ��
�״��ϳɷ�Ӧ��
(��)CO(g)��2H2(g)=CH3OH(g)��H1����90.1 kJ��mol��1
(��)CO2(g)��3H2(g)=CH3OH(g)��H2O(g)��H2����49.0 kJ��mol��1
ˮú���任��Ӧ��
(��)CO(g)��H2O(g)=CO2(g)��H2(g)��H3����41.1 kJ��mol��1
�����Ѻϳɷ�Ӧ��
(��)2CH3OH(g)=CH3OCH3(g)��H2O(g)��H4����24.5 kJ��mol��1
��H2��COֱ���Ʊ�������(��һ����Ϊˮ����)���Ȼ�ѧ����ʽΪ_______________��
���ݻ�ѧ��Ӧԭ������������ѹǿ��ֱ���Ʊ������ѷ�Ӧ��Ӱ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⡣
��1����¯ұ�������У������ڴ���Ӧ���в���ˮú����CO��H2����ԭ���������йط�ӦΪ��CH4��g����CO2��g��=2CO��g����2H2��g������H��260 kJ��mol��1
��֪��2CO��g����O2��g��=2CO2��g����H����566 kJ��mol��1��
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ____________________________________��
��2������ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ�أ��������ҺΪKOH��Һ����ͨ��װ�â�ʵ�������϶�ͭ��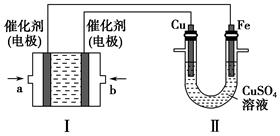
��a��Ӧͨ��________���CH4����O2������b���缫�Ϸ����ĵ缫��Ӧʽ��_________________________________________________________________��
�ڵ�ƽ�����װ�â�����Һ��pH________����д�������С�����䡱����ͬ����װ�â���Cu2�������ʵ���Ũ��________��
�۵�ƽ�����װ�â���Һ�е������ӳ���OH���������________������ˮ�⣩��
���ڴ˹���������ȫ��Ӧ��װ�â������������仯12.8 g����װ�â������������ļ���________L����״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һ�������£������Ϊ3 L���ܱ������з�Ӧ��CO��g��+ 2H2��g�� CH3OH��g���ﵽ��ѧƽ��״̬��
CH3OH��g���ﵽ��ѧƽ��״̬��
��1���÷�Ӧ��ƽ�ⳣ������ʽK= ��������ͼ�������¶ȣ�Kֵ�� �����������С�����䡱����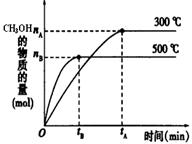
��2��500��ʱ���ӷ�Ӧ��ʼ���ﵽ��ѧƽ�⣬��H2��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ������ ����nB��tB��ʾ����
��3���жϸÿ��淴Ӧ�ﵽ��ѧƽ��״̬�ı�־�� ������ĸ����
a��CO��H2��CH3OH��Ũ�Ⱦ����ٱ仯
b�����������ܶȲ��ٸı�
c����������ƽ����Է����������ٸı�
d��v������CH3OH��= v������CO��
��4��300��ʱ�����������ݻ�ѹ����ԭ����1/2���������������������£���ƽ����ϵ������Ӱ���� ������ĸ����
a��c��H2������
b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c��CH3OH �����ʵ�������
d������ƽ��ʱc��H2��/ c��CH3OH����С
��5��������Ŀ�й���Ϣ��������������ͼ�б�ʾ���û�ѧ��Ӧ���̵������仯��������Ϣ����
��6���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ��2CH3OH +3O2+4OH- = 2CO32- + 6H2O���õ���и����ϵĵ缫��Ӧʽ�ǣ�2CH3OH�C12e��+16OH���� 2CO32��+ 12H2O ���������Ϸ����ĵ缫��ӦΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���º���������,����Է�������ת��,�䷴Ӧ���̺�������ϵ��ͼ1��ʾ����֪:2SO2(g)+O2(g) 2SO3(g)��
2SO3(g)��
��H="-196.6" kJ/mol��
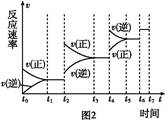
��ش���������:
(1)д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ: ��
(2)��H2=����������������������
(3)���º���ʱ,1 mol SO2��2 mol O2��ַ�Ӧ,�ų���������ֵ�ȨO��H2�O��������(�����С������ȡ�)��
(4)�����еĻ������ͨ��������NaOH��Һ������NaOH�����ʵ���Ϊ��������,����Һ�з�����������ԭ��Ӧ,��ù��̵����ӷ���ʽΪ�� ��
(5)����������,���д�ʩ����ʹn(SO3)/ n(SO2)�����������������
a.�����¶�
b.����He��
c.�ٳ���1 mol SO2(g)��1 mol O2(g)
d.ʹ�ô���
(6)ijSO2(g)��O2 (g)��ϵ,ʱ��t1�ﵽƽ���,�ı�ijһ�������,��Ӧ����v��ʱ��t�Ĺ�ϵ��ͼ2��ʾ,�����ı�SO2(g)��O2 (g)����,��ͼ��t4ʱ����ƽ���ƶ���������������������;ͼ�б�ʾƽ��������SO3�ĺ�����ߵ�һ��ʱ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Դ�Ŀ����������������Ŀɳ�����չϢϢ��ء�
��.��֪��Fe2O3��s����3C��s��=2Fe��s����3CO��g��
��H1��a kJ��mol��1
CO��g����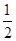 O2��g��=CO2��g���� ��H2��b kJ��mol��1
O2��g��=CO2��g���� ��H2��b kJ��mol��1
4Fe��s����3O2��g��=2Fe2O3��s���� ��H3��c kJ��mol��1
��C��ȼ���Ȧ�H��________kJ��mol��1��
��.��1������ԭ��صĹ���ԭ�������л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���________������ţ���
A��C��s����CO2��g��=2CO��g��
B��NaOH��aq����HCl��aq��=NaCl��aq����H2O��l��
C��2H2O��l��=2H2��g����O2��g��
D��2CO��g����O2��g��=2CO2��g��
�������ڵ�K2CO3��CO2Ϊ��Ӧ�Ļ�����������ѡ��Ӧ��Ƴ�һ��ԭ��أ���д����ԭ��صĸ�����Ӧ��_____________________________________��
��2��ijʵ��С��ģ�ҵ�ϳɰ���ӦN2��g����3H2��g�� 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��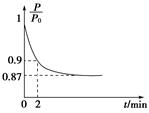
��ش��������⣺
�ٷ�Ӧ��ƽ��ı�־��__________________________������ĸ���ţ���ͬ����
A��ѹǿ���ֲ���
B�������ܶȱ��ֲ���
C��NH3������������N2���������ʵ�2��
��0��2 min�ڣ���c��N2���仯��ʾ��ƽ����Ӧ����Ϊ________________��
�������N2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_____________________________��
A������ϵ�а������1��1�ٳ���N2��H2
B�������NH3
C�������¶�
D�����뺤��ʹѹǿ����
E������һ������N2
��3��25��ʱ��BaCO3��BaSO4���ܶȻ������ֱ���8��10��9��1��10��10��ij����BaCO3����������Һ�У�c��CO32-����0.2 mol��L��1���������������Na2SO4��Һ����Ҫ����BaSO4����������Na2SO4��Һ�����ʵ���Ũ����С��________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��Ӧ��Fe(s)+CO2(g) FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g)
FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g) CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g)
CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |
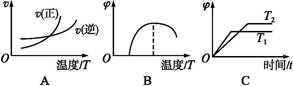
 2FeO(s)�Ħ�H=����������
2FeO(s)�Ħ�H=���������� �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com