



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
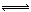 H++A2-����֪������Ũ��Ϊ0.1mol/L��H2A��Һ��c(H+)��0.11mol/L�������0.1mol/L��NaHA��Һ��������������ȷ���ǣ��� ��
H++A2-����֪������Ũ��Ϊ0.1mol/L��H2A��Һ��c(H+)��0.11mol/L�������0.1mol/L��NaHA��Һ��������������ȷ���ǣ��� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������� | B������NaOH | C������KOH | D������KHS |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(Na+)��c(CH3COO��)��c(H+)>c(OH��) | B��c(Na+)��c(CH3COO��)��c(OH��)��c(H+) |
| C��c(Na+)��c(CH3COO��)+c(CH3COOH)�� | D��c(Na+)+c(H+)��c(CH3COO��)+c(OH��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
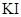 ��Ϊ0.01mol/L����Һ�м���8mL0.01mol/L��AgNO3��Һ,��ʱ��Һ���������ʵ�����Ũ�ȴ�С��ϵ��ȷ����
��Ϊ0.01mol/L����Һ�м���8mL0.01mol/L��AgNO3��Һ,��ʱ��Һ���������ʵ�����Ũ�ȴ�С��ϵ��ȷ���� | A��C(K+)>C(NO3-)>C(Cl-)>C(Ag+)>C(I-) |
| B��C(K+)>C(NO3-)>C(Ag+)> (Cl-)>(I-) |
| C��C(NO3-)>(K+)> C(Ag+)> (Cl-)>(I-) |
| D��(K+)> C(NO3-)>C(Ag+)=(Cl-)+(I-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 mol/L��Na2CO3��FeCl3��Һ����Na2CO3��Һ�е����̪������Һ����________(�dz�족��ɫ����ԭ��__________________________(�����ӷ��̱���)�����ȣ�����ɫ��_________(dz����)����FeCl3��Һ�е���ʯ�������Һ����________������ɫ�������������ɲ����գ���õ��Ĺ�������Ϊ��__________________________���������͵�FeCl3�����ˮ�������ķ�Ӧ�� _________________________(�����ӷ��̱���)
mol/L��Na2CO3��FeCl3��Һ����Na2CO3��Һ�е����̪������Һ����________(�dz�족��ɫ����ԭ��__________________________(�����ӷ��̱���)�����ȣ�����ɫ��_________(dz����)����FeCl3��Һ�е���ʯ�������Һ����________������ɫ�������������ɲ����գ���õ��Ĺ�������Ϊ��__________________________���������͵�FeCl3�����ˮ�������ķ�Ӧ�� _________________________(�����ӷ��̱���)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����<��<��<��<�١��� | B����<��<��<��<�١��� |
| C����<��<��<��<�� | D����<��<��<��<�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com