
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�
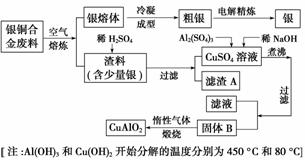
(1)��⾫����ʱ��������ӦʽΪ________������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ��Ӧ����ʽΪ________________��
(2)��������B�����Ϊ__________�������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ____________��
(3)������չ�����һ����Ӧ�Ļ�ѧ����ʽ��____CuO��____Al2O3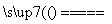 ____CuAlO2��________����
____CuAlO2��________����
(4)����ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0 kg�����е�ͭ����ȫת��Ϊ________ mol CuAlO2��������Ҫ1.0 mol��L��1��Al2(SO4)3��Һ________ L��
(5)CuSO4��ҺҲ�������Ʊ������������������________�����ˡ�ϴ�Ӻ��
�𰸡�(1)Ag����e��===Ag��2NO��O2===2NO2
(2)Al(OH)3��Cu(OH)2��Al(OH)3��OH��===AlO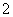 ��2H2O
��2H2O
(3)4��2��4��O2
(4)50��25
(5)�����ᾧ
���������ݵ��ԭ�������ʵ����ʼ��������̡�������ԭ��Ӧ���غ����ȷ�����
(1)��⾫����ʱ��������ӦʽΪAg����e��===Ag�������ɫ�����ķ�ӦΪ2NO��O2===2NO2��
(2)����B����Cu(OH)2��Al(OH)3����NaOH��������Al(OH)3��ת��ΪNaAlO2��
(3)�÷�ӦΪ������ԭ��Ӧ�����ݵ�ʧ�����غ㡢ԭ���غ�ȷ��ȱ�����ʣ�Ȼ����ƽ��ѧ����ʽ��
(4)����CuAlO2�����ʵ���Ϊ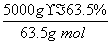 ��50 mol����AlԪ���غ��֪��������ҪAl2(SO4)3�����ʵ���Ϊ25 mol��������Һ���Ϊ25 L��
��50 mol����AlԪ���غ��֪��������ҪAl2(SO4)3�����ʵ���Ϊ25 mol��������Һ���Ϊ25 L��
(5)CuSO4�ӱ�����Һ�нᾧ����CuSO4��5H2O(����)����CuSO4��Һ�Ʊ�������Ҫ�IJ���Ϊ�����ᾧ�����ˡ�ϴ�Ӻ��


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�̨��������Һ
��pH���±���
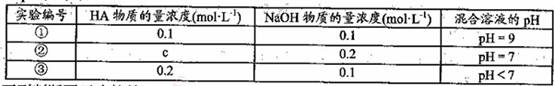
�����жϲ���ȷ����
A��HA�ĵ��뷽��ʽΪ��
B���������������ʵ������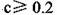
C������ʵ���У������Һ��
D������ʵ���У������Һ��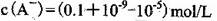
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У��������ۼ����� �� ��
����Cl2�� �£�NaCl �ã�HCl���� �ģ�NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ�����������Ԫ�أ�Ҳ����������ʹ�õĽ���֮һ��ͭ��������ʹ�öԹ��������������涼��������Զ��Ӱ�죬�����(1)��(3)�⣺
(1)�������ż������м��أ����������Ϊͭ[������(CuSO 4)������Ӧ����ͭ]����д���÷�Ӧ�Ļ�ѧ����ʽ��
4)������Ӧ����ͭ]����д���÷�Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(2)ͭ��������ʱ������ͭ�̣���д������ͭ�̵Ļ�ѧ����ʽ��________________________________________________________________________��
���ͭ�̿����û�ѧ������ȥ����д����ȥͭ�̶�����������Ļ�ѧ����ʽ��________________________________________________________________________��
(3)ͭǮ����ʷ��������һ�ֹ㷺��ͨ�Ļ��ҡ��Դ��������ʺͻ�ѧ���ʵĽǶȷ���Ϊʲôͭ������������ң�_____________________________________________________
________________________________________________________________________
________________________________________________________________________��
(ͭ���۵���1 183.4 �棬�����۵���1 534.8 ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йؽ����Ĺ�ҵ�Ʒ�����ȷ���� (����)
A�����ѣ��ý������û����Ȼ���(TiCl4)��Һ�е���
B���������ý�̿�Ϳ�����Ӧ������CO�ڸ����»�ԭ����ʯ������������
C�����ƣ��ú�ˮΪԭ���Ƶþ��Σ��ٵ�ⴿ����NaCl��Һ
D����ͭ���û�ͭ��⾫���õ�����Ϊ99.9%��ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ʊ��������ʵķ�����ԭ����ȷ���� (����)
A���ڸ��������£���H2��ԭMgO�Ʊ�����Mg
B����ͨ�������£��������Al2O3�Ʊ�����Al
C����ͨ�������£���ⱥ��ʳ��ˮ�Ʊ�����Na
D����ǿ�ȣ�ʹCuO�ڸ��������·ֽ��Ʊ�����Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס���Ϊ���ʣ�������Ϊ���������֮�������ͼ��ʾ��ת����ϵ��
(1)���ס��Ҿ�Ϊ�ǽ�������Ϊ�������
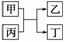 ����Ϊ��������ʱ���ҿ�����________(�ѧʽ����ͬ)������Ԫ�����ڱ��Ʋ⣬��ʱ��Ӧ�������__________________________________��
����Ϊ��������ʱ���ҿ�����________(�ѧʽ����ͬ)������Ԫ�����ڱ��Ʋ⣬��ʱ��Ӧ�������__________________________________��
����Ϊ��ԭ����ʱ�������������������___________________________________��
(2)����ת����ϵΪ�ҹ��Ŵ�ʪ��ұ�������ҵ�ԭ����д���˷�Ӧ�Ļ�ѧ����ʽ��__________________________________������ת����ϵΪ���»�ұ�������ҵ�ԭ������ͬʱ���ɵĶ���һ���д̼�����ζ�����壬�������Ϊ________________________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£������йش���������в���ȷ����(����)
A��pH��5.6��CH3COOH��CH3COONa�����Һ�У�c(Na��)<c(CH3COO��)
B��Ũ�Ⱦ�Ϊ0.1 mol��L��1��CH3COOH��Һ��CH3COONa��Һ�������Ϻ�c(CH3COO��)��c(CH3COOH)��2[c(H��)��c(OH��)]
C����pH��a�Ĵ���ϡ��ΪpH��a��1�Ĺ����У� ���ϼ�С
���ϼ�С
D�������pH��a�Ĵ�����pH��b��NaOH��Һǡ���к�ʱ��a��b��14
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�������£���CO��O2�Ļ������26g����һ����̶�������ܱ���������������������Na2O2���壩����ʱ������ѹǿΪp1,�õ����ϵĵ�ȼ��ʹ���ַ�Ӧ���ָ���ԭ�£������������14g����ʱ������ѹǿΪp2����p1/p2Ϊ
A��9��4 B��7��3 C��7��6 D��6��7
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com