
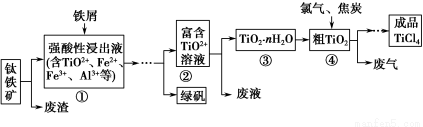
���Ȼ���(TiCl4)����ȡ���캽�չ�ҵ���ϡ����ѺϽ����Ҫԭ�ϡ���������(��Ҫ�ɷ���FeTiO3)�Ʊ�TiCl4�Ȳ�Ʒ��һ�ֹ�������ʾ�����£�
�ش��������⣺
(1)�����м�����м������Һ����ɫ����ʱ��Һ�Գ�ǿ���ԡ��ù����������·�Ӧ������
2Fe3����Fe=3Fe2��
2TiO2��(��ɫ)��Fe��4H��=2Ti3��(��ɫ)��Fe2����2H2O
Ti3��(��ɫ)��Fe3����H2O=TiO2��(��ɫ)��Fe2����2H��
������������� ��
(2)�ڢڡ��۹��չ�������Ҫ�����������γ�TiO2��nH2O�ܽ������ܽ��ķ�ɢ�ʿ���ֱ����С�� ��Χ��
(3)���Ѣ����ƵõĹ���TiO2��nH2O������ϴ��ȥ���е�Fe(OH)3���ʣ������Ƶ��Ѱۡ���֪25 ��ʱ��Ksp[Fe(OH)3]��2.79��10��39�����¶��·�ӦFe(OH)3��3H��??Fe3����3H2O��ƽ�ⳣ��K�� ��
(4)��֪��TiO2(s)��2Cl2(g)=TiCl4(l)��O2(g) ��H����140 kJ��mol��1
2C(s)��O2(g)=2CO(g)����H����221 kJ��mol��1
д������TiO2�ͽ�̿��������Ӧ����Һ̬TiCl4��CO������Ȼ�ѧ����ʽ�� ��
(5)�������վ��гɱ��͡����õ�Ʒλ����Ϊԭ�ϵ��ŵ㡣������ɫ��ѧ����ù��������д��ڵIJ���֮���� (ֻҪ��д��һ��)��
(6)�����±���Ϣ��Ҫ���ƺ�����SiCl4���ʵ�TiCl4���ɲ��� ������
| TiCl4 | SiCl4 |
�۵�/�� | ��25.0 | ��68.8 |
�е�/�� | 136.4 | 57.6 |
(1)ʹFe3����ԭΪFe2��������TiO2������Fe3������
(2)10��9 m��10��7 m(������������)
(3)2.79��103
(4)TiO2(s)��2Cl2(g)��2C(s)=TiCl4(l)��2CO(g)����H����81 kJ��mol��1��
(5)��������(������������)
(6)����(��������)
��������(1)����Ŀ�����ķ�Ӧ���Կ���������мʹ��Һ�е�Fe3��ת��ΪFe2��������TiO2������Fe3��������
(3)��Ksp[Fe(OH)3]��2.79��10��39�ɵ�c(Fe3��)��c3(OH��)��2.79��10��39
K��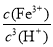 ��
��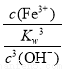 ��
��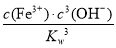
��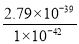 ��2.79��103��
��2.79��103��
(4)���ø�˹���ɣ������Ȼ�ѧ����ʽ��Ӽ�����Ҫ���Ȼ�ѧ����ʽTiO2(s)��2Cl2(g)��2C(s)=TiCl4(l)��2CO(g)����H����81 kJ��mol��1��
(5)�ɹ�ҵ����ͼ���Եó������������в����˹�ҵ���ϡ�
(6)TiCl4��SiCl4�ķе����ܴ����Բ�������ķ������з��롣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ���ͳ����ϰ �л������ɡ��ṹ�����ʣ������棩 ���ͣ�ѡ����
�������л���Ľṹ�������йص�������ȷ���ǣ� ��
A��������ʹ���Ը��������Һ��ɫ�����Ա����ܷ���������Ӧ
B��ʯ�͵���Ҫ�ɷ�������ú����������Ƶý�̿��ú���͵Ȳ�Ʒ
C����ϩ����������ԭ�Ӳ�������ͬһƽ����
D�����ۡ���ά�ض�����Ȼ�߷����л���������ж�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ���ͳ����ϰ ��ѧ������������ѧ��STSE���⣨�����棩 ���ͣ�ѡ����
��ѧ�뻷������Դ�����ϺͿƼ���������ء�����˵���в���ȷ���ǣ� ��
A�������������ҵ硰�Ծɻ��¡����������Դ����Ч�ʣ��뻷������������ϵ
B��ũ�������չ�������������Ľո�ת��Ϊ����Ч����Դ
C��þ���Ͻ����������ɻ��IJ���
D������ȼ�ϵ��Ҫ�㷺��Ӧ����ʵ�������������У�������������ϵ�����������
�Ĺؼ���������֮һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ�ս̰�ר��3���������ӹ��漰���ӷ���ʽ��д��ϰ���������棩 ���ͣ�ѡ����
�����������£�һ���ܴ���������������ǣ� ����
A����ɫ����ˮ��Һ�У�K����Ba2����Cl����MnO4��
B�����д���NO3����ˮ��Һ�У�NH4����Fe2����SO42����H��
C�������̪�Լ��Ժ�ɫ����Һ�У�Na����K����CO32����Br��
D��ǿ������Һ�У�ClO����S2����HSO3����Na��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ�ս̰�ר��3���������ӹ��漰���ӷ���ʽ��д��ϰ���������棩 ���ͣ�ѡ����
���и���������ָ����Һ���ܴ���������ǣ� ����
A��ʹ��̪���ɫ����Һ��Fe3����Mg2����SO42����NO3��
B��KNO3��������Һ��Fe2����Ca2����Al3����Cl��
C�������£���ˮ�������c(H��)��1.0��10��10 mol��L��1����Һ��NH4����Na����SiO32����CO32��
D��������Һ��Cu2����Fe3����NO3����MnO4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ�ս̰�һ�ָ�ϰר��2 �����ӵ��������ж���ϰ���������棩 ���ͣ�ѡ����
��NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ� ����
A�����³�ѹ�£�5.6 g��ϩ�ͻ�����Ļ�������к��е�̼ԭ����Ϊ0.4 NA
B��1 mol Cl2ͨ������ˮ�з�Ӧת�Ƶĵ�����ΪNA
C��0.1 mol��L��1 Na2CO3��Һ��CO32����HCO3����H2CO3����Ϊ0.1 NA
D����״���£�2.24 L���ȼ����к���C��Cl��ĿΪ0.3 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧ�ս̰�һ�ָ�ϰר��2 �����ӵ��������ж���ϰ���������棩 ���ͣ�ѡ����
����˵����ȷ���ǣ� ����
A��3.9 g Na2O2������������������Ϊ0.2NA�������Ĺ��ۼ���Ϊ0.05NA
B��14 g C2H4��C3H6�Ļ�����У�����Hԭ����Ϊ2NA
C��PCl3��NO2��BF3��HClO��Щ������ÿ��ԭ�Ӷ��ﵽ��8�����ȶ��ṹ
D����⾫��ͭʱ������������3.2 gͭʱ��ת�Ƶĵ�����Ϊ0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ³�ư�ѡ���ĵ�3�� ������ˮ��Һ�е���Ϊ��ϰ���������棩 ���ͣ������
���ֽⷴӦ����ѧ��ѧ�г�����һ�ַ�Ӧ���͡�
(1)��֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1 mol��L��1������6����Һ��pH(C6H5OH�൱��һԪ����)��
���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | C6H5ONa |
pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 | 11.3 |
���ֽ��������һ�����ɣ�һ�ֽ�ǿ������һ�ֽ�������ο����Է��ط�Ӧ�����ɽ�����ͽ�ǿ����Σ��磺2CH3COOH��Na2CO3=2CH3COONa��CO2����H2O���������Ƕȿ�����ͬʱ��ʾ����һ�����ɣ������Խ�ǿ�����ʷ������Ʒ�Ӧ�������ɼ��Խ��������ʡ����ոù��ɣ����ж����з�Ӧ���ܳ�������______________(����)��
A��CO2��H2O��2NaClO=Na2CO3��2HClO
B��CO2��H2O��NaClO=NaHCO3��HClO
C��CO2��H2O��C6H5ONa�D��NaHCO3��C6H5OH
D��CO2��H2O��2C6H5ONa�D��Na2CO3��2C6H5OH
E��Na2CO3��C6H5OH�D��NaHCO3��C6H5ONa
F��CH3COOH��NaCN=CH3COONa��HCN
(2)����ǰ����Ϣ�жϣ�Ũ�Ⱦ�Ϊ0.05 mol��L��1�������������ʵ���Һ�У�pH��С����________(����)����pH����________(����ֵ)��pH������________(����)��
��C6H5OH����CH3COOH����HCN����HClO
��H2SO4����HClO4
(3)һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɡ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ
�ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ���
������KCl��NaNO3�����Һ����������NaCl�������������Ӧ���ܽ�����ֽⷴӦ��������һ����___________________________________________
��KI��Һ��AgCl�����Ͻ��裬��۲쵽��������____________________________________����д����Ӧ�����ӷ���ʽ______________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ³�ư�ѡ���ĵ�1�� ��ѧ��Ӧ������ת����ϰ���������棩 ���ͣ�ѡ����
�ҹ�ij����н����ļ���ν������ꡣ�ݻ������Ųⶨ���ó��������ļ������pHƽ��Ϊ3.2�������ֻ����е�����Ʒ���ױ���ʴ���Դ����������ĸ�ʴ����������ȷ����(����)
A���˸�ʴ�����л�ѧ��ʴҲ�е绯ѧ��ʴ
B�������绯ѧ��ʴʱ��������ӦΪ
2H2O��O2��4e��=4OH��
C���ڻ�ѧ��ʴ����������������
D�������绯ѧ��ʴʱ�ĸ�����ӦΪFe��2e��=Fe2��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com