

| A�� | ���е����ӽ���Ĥ��ѡ�������ӽ���Ĥ | |
| B�� | �����ĵ缫��ӦʽΪ��2HSO3-+2e-=S2O42-+2OH- | |
| C�� | �����ĵ缫��ӦʽΪ��SO2+2H2O-2e-=SO42-+4H+ | |
| D�� | ��Ӧһ��ʱ����Ե缫�������缫���Ե缫���ﲻ����Ӱ�� |
���� ����ͼʾ��֪��������������Ϊ����������Զ����������ڵ���Ϊ��������������������ӦSO2-2e-+2H2O�TSO42-+4H+���������Դ������a������bΪ��Դ�����������ĵ缫��ӦʽΪ��2HSO3-+2H++2e-�TS2O42-+2H2O��������ӵ��ƶ��������ķ���������
��� �⣺A������������ӦSO2-2e-+2H2O�TSO42-+4H+��������Ӧ��Ҫ����H+�������е����ӽ���ĤӦѡ�������ӽ���Ĥ����A����
B��bΪ��Դ���������Դ���������ĵ缫Ϊ������������ӦΪHSO3-�õ���������S2O42-���������ĵ缫��ӦʽΪ��2HSO3-+2H++2e-�TS2O42-+2H2O����B����
C���������Դ������a������������ӦΪSO2�õ����ӣ�����SO42-���������ĵ缫��ӦʽΪ��SO2+2H2O-2e-=SO42-+4H+����C��ȷ��
D��Fe�缫�������Ե缫����û��Ӱ�죬����Ϊ��������Ϊ���Ե缫���ᷢ���缫��Ӧ��Ӱ��缫�����D����
��ѡC��
���� ���⿼���˵��ԭ���ķ���Ӧ�ã���Ҫ�ǵ缫��Ӧ���缫�����жϣ��������ԭ���ǹؼ�����Ŀ�Ѷ��еȣ����е��⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢ� | B�� | �٢ڢ� | C�� | �ڢܢ� | D�� | �٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �¶ȸߵ��������ܲ�һ��������ƽ������һ���� | |
| B�� | ������������Ϊ����ʱ��������������Ӽ����ļ�С������ | |
| C�� | ����������������������һ������ | |
| D�� | �����Ӽ�ľ�������ʱ��������һ����С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʱ�䣨min�� Ũ�ȣ�mol•L-1�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1.00 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
| N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
| CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��Ӧ��� | CO2�����ʵ���/mol | NaOH��Һ�����/L | �ų�������/KJ |
| 1 | 0.5 | 0.75 | x |
| 2 | 1.0 | 2.00 | y |
| �¶� t/min | 0 | 40 | 80 | 120 | 160 |
| �ף�673K�� | 2.00 | 1.50 | 1.10 | 0.80 | 0.80 |
| �ң�T�� | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
| ����673K�� | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ${\;}_{53}^{131}$I�Ļ�ѧ������${\;}_{53}^{127}$��ͬ | |
| B�� | ${\;}_{53}^{131}$I��ԭ������Ϊ53 | |
| C�� | ${\;}_{53}^{131}$I�ĺ��������Ϊ78 | |
| D�� | ${\;}_{53}^{131}$Iԭ�Ӻ������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O | B�� | NaOH | C�� | H2 | D�� | Na2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2 | B�� | HCl | C�� | HClO | D�� | KClO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH2- | B�� |  | C�� | 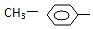 | D�� | R-CO- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com