
| c(��)��V(��) |
| V(����) |
| c(��)��V(��) |
| V(����) |
| c(��)��V(��) |
| V(����) |
| c(��)��V(��) |
| V(����) |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na+��Br-��CO32- |
| B��Na+��I-��SO32- |
| C��Fe2+��I-��SO32- |
| D��Fe2+��Br-��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2.4g |
| B��10.08g |
| C��3.36g |
| D��5.60g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
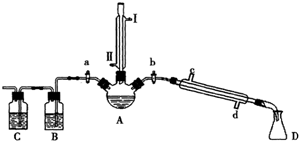
| ���� | �۵�/�� | �е�/�� |
| 1-���� | -89.5 | 117.3 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������̼�ᱵ��Ӧ CO32-+2H+�TCO2��+H2O |
| B��̼������ڴ��CaCO3+2H+=Ca2++H2O+CO2�� |
| C����������Һ�м���ͭ�� 2Ag++Cu�TCu2++2Ag�� |
| D������ϡ���ᷴӦ 2Fe+6H+�T2Fe3++3H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
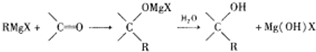
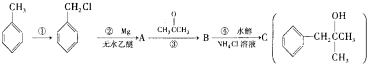
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ᡢKSCN��Һ��Fe��NO3��2��Һ������Һ��ϣ������Һ�Ժ�ɫ |
| B��SO2ͨ��Fe2��SO4��3��Һ�У����������� |
| C��SO2ͨ�����ữ��Ba��NO3��2��Һ�У����ְ�ɫ���� |
| D���ڵ��з�̪��Na2CO3��Һ�У�����BaCl2��Һ���ɫ��ȥ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������� |
| B��1H��2H |
| C����������� |
| D���Ҵ���CH3CH2OH���ͼ��ѣ�CH3OCH3�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com