
ˮ��һ�ּ�����Ҫ�Ļ�ѧ�Լ����ɲ�����ַ�Ӧ�������з�Ӧ�������һ������д����Ӧ�Ļ�ѧ����ʽ��
(1)ˮ��������____________��
(2)ˮ����ԭ��____________��
(3)ˮ�Ȳ���������Ҳ������ԭ����������ԭ��Ӧ____________��
(4)ˮ�Ȳ����������ֲ�����ԭ���ķ�������ԭ��Ӧ____________��
(5)ˮ����������____________��
(6)ˮ�ǻ�ԭ����____________��
(7)ˮ�Ȳ������������ֲ��ǻ�ԭ�����������ԭ��Ӧ____________��
(8)ˮ�Ƿ�������ԭ��Ӧ�IJ���____________��
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ѡ����
��ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����ѧʵ���ڻ�ѧѧϰ�о��м�����Ҫ�����á�
(1) ���й���ʵ���������ȷ����_______________________��
A��ȼ�ŵľƾ��Ʋ�������ʧ��Ӧ������ʪ������
B���ζ�ʵ�����õ���Һ�ܡ���ƿ�͵ζ��ܶ�Ҫ����ʢ��Һ��ϴ
C�������ȩ��֬ʵ����Թܿ����Ҵ����ݺ���ϴ��
D�����Ը��������Һһ���������ữ���ɳ�ȥ��ϩ�л��еĶ�����������
E����ȥ�Ȼ�þ��Һ�к��е�FeCl3 ����, �ɲ��ü���MgO����pH��ȥ��
F����������ʱ���ò��������Ͻ��裬��ֹҺ��ɽ���������ˮ����ȫ���ɺ�ֹͣ����
G��������Һ����ȡ0.10 mol/L ��KMnO4��Һ25.10 mL
H. ��pH��ֽ���ij������ˮ��pHֵΪ3
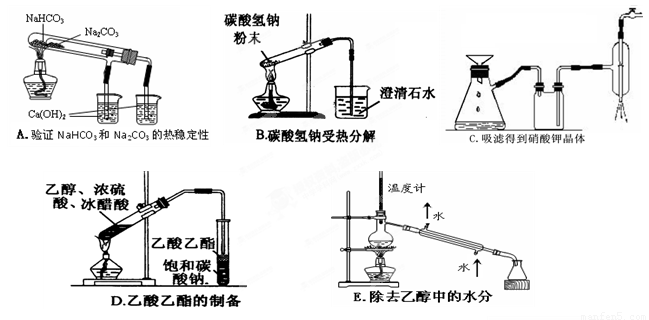 (2 ) ����ʵ��û�д������_______________________
(2 ) ����ʵ��û�д������_______________________
(3) ��ͼΪ���������IJ��ֽṹ���е��������Ŵ�
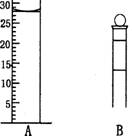
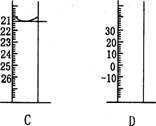
Aͼ��Һ����ʾ��Һ�����Ϊ__________mL�����������������е�ij�ֲ���һҺ�������� ƽ��ʱ����ΪNmL������ʱ����ΪM mL����M>N������ʹ�õ�������________������ĸ��ţ���
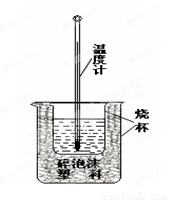
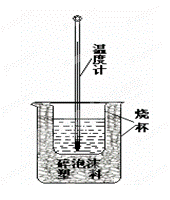
(4)���к��ȵIJⶨ����ͼ��ʾ����װ��ͼ��������ʵ����Ʒû�л������������ձ��Ϸ�����ĭ���ϸǺ�_________________�����һ���к��Ȳⶨʵ�飬�¶ȼ�����Ҫʹ�� �Ρ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
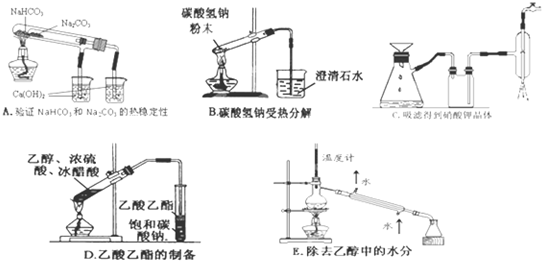
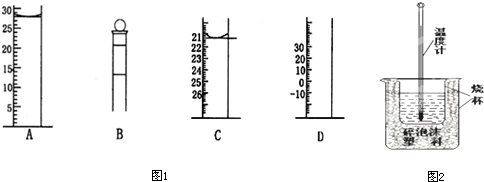
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ����һ�и߶����£���ĩ��ѧ�Ծ��������棩 ���ͣ������

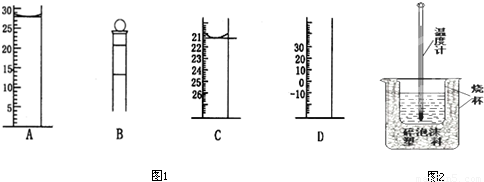
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ����һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ���ѡ��
��ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����ѧʵ���ڻ�ѧѧϰ�о��м�����Ҫ�����á�
(1) ���й���ʵ���������ȷ����_______________________��
| A��ȼ�ŵľƾ��Ʋ�������ʧ��Ӧ������ʪ������ |
| B���ζ�ʵ�����õ���Һ�ܡ���ƿ�͵ζ��ܶ�Ҫ����ʢ��Һ��ϴ |
| C�������ȩ��֬ʵ����Թܿ����Ҵ����ݺ���ϴ�� |
| D�����Ը��������Һһ���������ữ���ɳ�ȥ��ϩ�л��еĶ����������� |
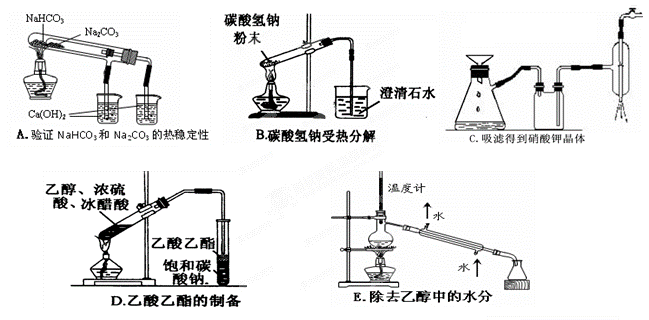 (2 ) ����ʵ��û�д������_______________________
(2 ) ����ʵ��û�д������_______________________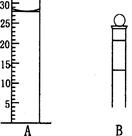

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com