

 ____������G��______________________��
____������G��______________________�� (4)��ʵ���β���Ƿ��账����_______�����账������ش���δ������粻�账����
(4)��ʵ���β���Ƿ��账����_______�����账������ش���δ������粻�账����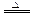 3Fe��4CO������F
3Fe��4CO������F e3O4��2C
e3O4��2C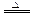 3Fe��2CO2����
3Fe��2CO2����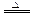 3Fe��4CO2�� ��1�֣�
3Fe��4CO2�� ��1�֣� �֣�
�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 ��
�� 
 ��3���ӻ��������ĽǶȿ��ǣ���
��3���ӻ��������ĽǶȿ��ǣ��� ��Ϊ��ʵ�����Ƹ���θĽ�?��д��һ�ָĽ�����:
��Ϊ��ʵ�����Ƹ���θĽ�?��д��һ�ָĽ�����:�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ҵ��е����ᣨ��ʯ�ң����� |
| B�������е���ϩ��NaOH��Һ��ϴ���� |
| C���屽�е��壨KI��Һ����Һ�� |
| D�����������е����ᣨ����NaOH��Һ�����ˣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ױ�����������KMnO4��Һ�����Һ |
| B�����飨��ϩ����ͨ��ʢ����ˮ��ϴ��ƿ |
| C���Ҵ��������������÷�Һ©����Һ |
| D���������ӣ�����Ũ��ˮ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ˮ | B��������ͱ� | C���屽��ˮ | D���Ҵ���ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��1������һ�����ʵ���Ũ�ȵ�BaC12��Һ�����Ѿ������õ� gBaC12��������0.4mol��L��lBaCl2��Һ250 mL������Ҫ������Ϊ��Ͳ�� ��
��1������һ�����ʵ���Ũ�ȵ�BaC12��Һ�����Ѿ������õ� gBaC12��������0.4mol��L��lBaCl2��Һ250 mL������Ҫ������Ϊ��Ͳ�� ��
 ʵ���д���ƿ�в��ٲ�����������ӵ���A�˻�������һ�����Ŀ�������������Ŀ���� ��
ʵ���д���ƿ�в��ٲ�����������ӵ���A�˻�������һ�����Ŀ�������������Ŀ���� �� ���� ��
���� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO2ͨ�����Ը��������Һ�У���Һ��ɫ��˵��SO2����Ư���� |
| B������NaOH��Һ�����ɵİ�ɫ�����ڿ�����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ����ԭ��Һ��һ������Fe2+ |
| C������Ba(NO3)2��Һ�а�ɫ�����������ټ����ᣬ��������ʧ����ԭ��Һһ����SO42- |
| D���ýྻ�IJ�˿պȡ��Һ�ڻ��������գ�������ɫ�Ļ��棬��ԭ��Һ��һ��������K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 aCl��Һ(NaHCO3)���Լ� �����ӷ���ʽ ��
aCl��Һ(NaHCO3)���Լ� �����ӷ���ʽ ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com