
 ��
�� ��
������ ��1���·���ʽΪNH2-NH2��ÿ����ԭ���γ�������ѧ���������������ʽH2O2��ÿ����ԭ���γ��������ۼ������ݽṹд�����ʵĽṹʽ��
��2����ȼ�����ɵ�����ˮ��
��3�������Ȼ�ѧ����ʽ��д����д������ע���ʾۼ�״̬�ͷ�Ӧ�ʱ䣻
��4�����ݢ�N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.6kJ/mol��
��H2O��l���TH2O��g����H=+44kJ/mol��
���ݸ�˹���ɣ���-�ڡ�4�õ���N2H4��l��+2H2O2��l���TN2��g��+4H2O��l����H=-817.6kJ/mol���ݴ˼��㣻
��5����N2��g��+2O2��g���T2NO2��g������H=+67.7KJ/mol��
��N2H4��g��+O2��g���TN2��g��+2H2O��g������H=-534KJ/mol��
���ݸ�˹���ɢڡ�2-�ٵõ�����NO2��ȫ��Ӧ���Ȼ�ѧ����ʽ��
��� �⣺��1���·���ʽΪNH2-NH2��ÿ����ԭ���γ�������ѧ�����ṹʽΪ�� �������������ʽH2O2��ÿ����ԭ���γ��������ۼ������ӽṹΪ��
�������������ʽH2O2��ÿ����ԭ���γ��������ۼ������ӽṹΪ�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��2����ȼ�����ɵ�����ˮ�����ͷŴ����ȺͿ��ٲ������������⣬���ɵ���������Ⱦ��
�ʴ�Ϊ������N2��H2O���Ի�������Ⱦ��
��3��0.4molҺ̬���������������ⷴӦ�����ɵ�����ˮ�������ų�256.65kJ��������32g��ȼ�շ���641.6kJ����ȼ�յ��Ȼ�ѧ����ʽΪ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.6kJ/mol��
�ʴ�Ϊ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.6kJ/mol��
��4����N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.6kJ/mol��
��H2O��l���TH2O��g����H=+44kJ/mol��
���ݸ�˹���ɣ���-�ڡ�4�õ���N2H4��l��+2H2O2��l���TN2��g��+4H2O��l����H=-817.6kJ/mol
��16gҺ̬��������Һ̬�������ⷴӦ���ɵ�����Һ̬ˮ����ʱ408.8KJ��
�ʴ�Ϊ��408.8KJ��
��5����֪��N2��g��+2O2��g���T2NO2��g������H=+67.7KJ/mol��
��N2H4��g��+O2��g���TN2��g��+2H2O��g������H=-534KJ/mol��
���ݸ�˹���ɢڡ�2-�ٵõ�����NO2��ȫ��Ӧ���Ȼ�ѧ����ʽ��2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1135.7KJ/mol��
�ʴ�Ϊ��2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1135.7KJ/mol��
���� ���⿼���˻�ѧ��Ӧ�����仯�ļ���Ӧ�ã����ʽṹ�����жϣ���˹���ɵļ��㣬�Ȼ�ѧ����ʽ��д��������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
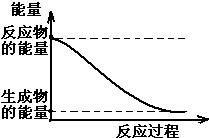 ��1��20����30�����Eyring��Pzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ���������Ĺ���̬����ͼ��NO2��CO��Ӧ����CO2��NO�����е������仯ʾ��ͼ��˵�������Ӧ�Ƿ��ȣ�������ȡ����ȡ�����Ӧ��NO2��CO�����������ڣ���
��1��20����30�����Eyring��Pzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ���������Ĺ���̬����ͼ��NO2��CO��Ӧ����CO2��NO�����е������仯ʾ��ͼ��˵�������Ӧ�Ƿ��ȣ�������ȡ����ȡ�����Ӧ��NO2��CO�����������ڣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | [Ne]3s1[Ne]3s2 | B�� | [Ar]4s1[Ne]3s23p4 | ||
| C�� | [Ne]3s2[Ar]4s2 | D�� | [He]2s22p4[Ne]3s23p5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | �� | �� | ||||||
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | ||||
| 4 | �� | �� |
 �����������ոû��������ʻ�ɫ��
�����������ոû��������ʻ�ɫ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Rλ��Ԫ�����ڱ��еĵ�VA�� | B�� | Rλ��Ԫ�����ڱ��еĵ� VIIA�� | ||
| C�� | RO3-�е�RԪ��ֻ�ܱ���ԭ | D�� | R2�ڳ��³�ѹ��һ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���й��ۼ��Ļ�����һ���ǹ��ۻ����� | |
| B�� | �ڹ��ۻ�������һ�����й��ۼ� | |
| C�� | �������Ӽ��Ļ�����һ�������ӻ����� | |
| D�� | ȫ���ɷǽ�����ɵĻ�������������ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com