
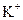 Ӣ
Ӣ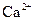 Ӣ
Ӣ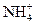 Ӣ
Ӣ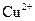 Ӣ
Ӣ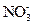 Ӣ
Ӣ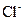 Ӣ
Ӣ Ӣ
”¢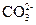 °ĖÖÖĄė×ÓÖŠµÄČōøÉÖÖ”£ĪŖČ·¶Ø¼×ČÜŅŗµÄ×é³É£¬½«Ęä·Ö³ÉĮ½µČ·Ż£¬½ųŠŠČēĻĀŹµŃé£ŗ
°ĖÖÖĄė×ÓÖŠµÄČōøÉÖÖ”£ĪŖČ·¶Ø¼×ČÜŅŗµÄ×é³É£¬½«Ęä·Ö³ÉĮ½µČ·Ż£¬½ųŠŠČēĻĀŹµŃé£ŗ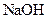 ÅØČÜŅŗ²¢¼ÓČČ£¬²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀĢå»żĪŖ4.48L”£
ÅØČÜŅŗ²¢¼ÓČČ£¬²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀĢå»żĪŖ4.48L”£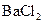 ČÜŅŗ500mL£¬Ē”ŗĆæÉŅŌÓėČÜŅŗÖŠµÄĄė×ÓĶźČ«·“Ó¦£¬¹żĀĖµĆ66.3g³Įµķ¼°ĀĖŅŗ”£
ČÜŅŗ500mL£¬Ē”ŗĆæÉŅŌÓėČÜŅŗÖŠµÄĄė×ÓĶźČ«·“Ó¦£¬¹żĀĖµĆ66.3g³Įµķ¼°ĀĖŅŗ”£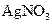 ČÜŅŗ650mL£¬Ē”ŗĆæÉĶźČ«·“Ó¦”£¾Ż“Ė£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ØÓĆĻąÓ¦µÄĄė×Ó·ūŗűķŹ¾£©£ŗ
ČÜŅŗ650mL£¬Ē”ŗĆæÉĶźČ«·“Ó¦”£¾Ż“Ė£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ØÓĆĻąÓ¦µÄĄė×Ó·ūŗűķŹ¾£©£ŗ ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 Ģ壬¼ÓČėNa2O2µÄĪļÖŹµÄĮæÓėĪö³ö³ĮµķµÄĪļÖŹµÄĮæČēĶ¼ĖłŹ¾”£ŹŌĶʶĻ£ŗ
Ģ壬¼ÓČėNa2O2µÄĪļÖŹµÄĮæÓėĪö³ö³ĮµķµÄĪļÖŹµÄĮæČēĶ¼ĖłŹ¾”£ŹŌĶʶĻ£ŗ
 ӣ
ӣ _ ӣ
_ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Na+”¢Cu2+”¢CO32£”¢Cl£ |
| B£®Na+”¢ Cl£”¢ HCO3£”¢Mg2+”” |
| C£®Cl£”¢NO3£”¢K+”¢ Ca2+”” |
| D£®NH4+”¢ K+”¢ H+”¢ SO42£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÓĆĻ”ĻõĖįĻ“µÓ×öŅų¾µ·“Ó¦µÄŹŌ¹Ü£ŗ3Ag +4 H++ NO3¯”ś3Ag++ NO”ü+ 2H2O |
| B£®ÓĆĒāŃõ»ÆÄĘČÜŅŗ³żČ„ĀĮ±ķĆęµÄŃõ»ÆĤ£ŗAl(OH)3 + OH¯”śAlO2¯+ 2H2O |
| C£®ÓĆĖ«ŃõĖ®ŗĶĻ”ĮņĖįČܽāÓ”Ė¢µēĀ·Ķ°å£ŗCu£«H2O2£«2H+”śCu2+£«2H2O |
| D£®ÓĆŹ³“׳żČ„Ė®ĘæÖŠµÄĖ®¹ø£ŗCO32¯+ 2CH3COOH ”ś2CH3COO¯+ CO2”ü+ H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Na+ ”¢I-”¢NO3-”¢ClO- | B£®Na+”¢0H- ”¢NO3-”¢HCO3- |
| C£®H+”¢Fe2+”¢NO3-”¢Cl- | D£®[Al(OH)4]-”¢SO42-”¢0H-”¢Na+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

| A£®Cu2+”¢Na+”¢SO42£”¢Cl£ | B£®K+”¢Na+”¢HCO3£”¢NO3£ |
| C£®OH£”¢HCO3£”¢Ca2+”¢Na+ | D£®Ba2+”¢Na+”¢Cl£”¢NO3£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ca2+”¢AlO2-”¢SO42-”¢Cl- | B£®K+”¢Na+”¢ClO-”¢Cl- |
| C£®Na+”¢Fe2+”¢NO3-”¢SO42- | D£®NH4+”¢Na+”¢F-”¢CO32- |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com