
£Ø9·Ö£¬ĆææÕ1·Ö£©Ä³æĪĶā»ī¶ÆŠ”×é¶Ō¼×Ėį½ųŠŠĮĖČēĻĀµÄŹµŃ飬ŅŌŃéÖ¤Ęäŗ¬ÓŠČ©»ł£¬²¢æ¼²ģĘä»ÆѧŠŌÖŹ£¬Ź×ĻČ×öĮĖŅų¾µ·“Ó¦.
£Ø1£©ŌŚ¼×Ėį½ųŠŠŅų¾µ·“Ó¦Ē°£¬±ŲŠėŌŚĘäÖŠ¼ÓČėŅ»¶ØĮæµÄ £¬ŅņĪŖ £»
£Ø2£©Š“³ö¼×Ėį½ųŠŠŅų¾µ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø3£©Ä³Ķ¬Ń§×öŅų¾µ·“Ó¦Ź§°Ü£¬ĖūæÉÄܽųŠŠµÄ“ķĪó²Ł×÷ÓŠ__ ______£ØŠ“×ÖÄø£©£ŗ
A£®ÓĆ½ą¾»µÄŹŌ¹Ü£»B£®ŌŚÅضČĪŖ2%µÄNH3”¤H2OÖŠµĪČėÉŌ¹żĮæµÄÅضČĪŖ2%µÄĻõĖįŅų£»
C£®ÓĆĮŁŹ±ÅäÖĘŗƵÄŅų°±ČÜŅŗ£»D£®ŌŚŅų°±ČÜŅŗĄļ¼ÓČėÉŌ¹żĮæµÄ¼×Ėį£»
E£®·“Ó¦¹ż³ĢÖŠ£¬Ć»ÓŠÕńµ“ŹŌ¹Ü.
Č»ŗó£¬Ķ¬Ń§ĆĒ¶Ō¼×ĖįÓė¼×“¼½ųŠŠĮĖõ„»Æ·“Ó¦µÄŃŠ¾æ£ŗ
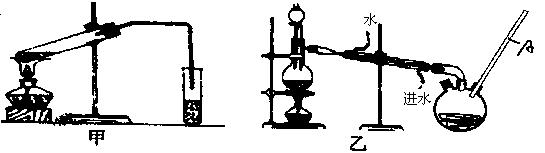
£Ø4£©ŅŅ×°ÖĆÖŠ³¤µ¼¹ÜAµÄ×÷ÓĆŹĒ £»
£Ø5£©Ń”Ōń¼××°ÖĆ»¹ŹĒŅŅ×°ÖĆŗĆ£æ________£¬ŌŅņŹĒ £»
£Ø6£©ŹµŃé¹ż³ĢÖŠŃ”ÓƵÄŅ©Ę·¼°ŹŌ¼ĮÓŠ£ŗÅØH2SO4”¢¼×“¼”¢¼×Ėį»¹ÓŠ________”¢________Į½ÖÖ±Ų±øÓĆĘ·.

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø9·Ö£¬ĆææÕ1·Ö£©Ä³æĪĶā»ī¶ÆŠ”×é¶Ō¼×Ėį½ųŠŠĮĖČēĻĀµÄŹµŃ飬ŅŌŃéÖ¤Ęäŗ¬ÓŠČ©»ł£¬²¢æ¼²ģĘä»ÆѧŠŌÖŹ£¬Ź×ĻČ×öĮĖŅų¾µ·“Ó¦.
£Ø1£©ŌŚ¼×Ėį½ųŠŠŅų¾µ·“Ó¦Ē°£¬±ŲŠėŌŚĘäÖŠ¼ÓČėŅ»¶ØĮæµÄ £¬ŅņĪŖ £»
£Ø2£©Š“³ö¼×Ėį½ųŠŠŅų¾µ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø3£©Ä³Ķ¬Ń§×öŅų¾µ·“Ó¦Ź§°Ü£¬ĖūæÉÄܽųŠŠµÄ“ķĪó²Ł×÷ÓŠ__ ______£ØŠ“×ÖÄø£©£ŗ
A£®ÓĆ½ą¾»µÄŹŌ¹Ü£»B£®ŌŚÅضČĪŖ2%µÄNH3”¤H2OÖŠµĪČėÉŌ¹żĮæµÄÅضČĪŖ2%µÄĻõĖįŅų£»
C£®ÓĆĮŁŹ±ÅäÖĘŗƵÄŅų°±ČÜŅŗ£»D£®ŌŚŅų°±ČÜŅŗĄļ¼ÓČėÉŌ¹żĮæµÄ¼×Ėį£»
E£®·“Ó¦¹ż³ĢÖŠ£¬Ć»ÓŠÕńµ“ŹŌ¹Ü.
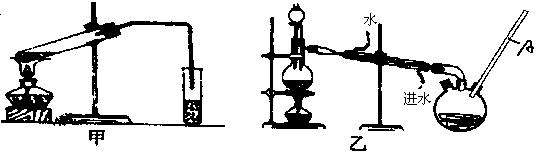
Č»ŗó£¬Ķ¬Ń§ĆĒ¶Ō¼×ĖįÓė¼×“¼½ųŠŠĮĖõ„»Æ·“Ó¦µÄŃŠ¾æ£ŗ
£Ø4£©ŅŅ×°ÖĆÖŠ³¤µ¼¹ÜAµÄ×÷ÓĆŹĒ £»
£Ø5£©Ń”Ōń¼××°ÖĆ»¹ŹĒŅŅ×°ÖĆŗĆ£æ________£¬ŌŅņŹĒ £»
£Ø6£©ŹµŃé¹ż³ĢÖŠŃ”ÓƵÄŅ©Ę·¼°ŹŌ¼ĮÓŠ£ŗÅØH2SO4”¢¼×“¼”¢¼×Ėį»¹ÓŠ________”¢________Į½ÖÖ±Ų±øÓĆĘ·.
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ£Ø½āĪö½ĢŹ¦×ØÓĆ£©2010Äź½Ī÷Ź”ÉĻø߶žÖŠø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗŹµŃéĢā
£Ø9·Ö£¬ĆææÕ1·Ö£©Ä³æĪĶā»ī¶ÆŠ”×é¶Ō¼×Ėį½ųŠŠĮĖČēĻĀµÄŹµŃ飬ŅŌŃéÖ¤Ęäŗ¬ÓŠČ©»ł£¬²¢æ¼²ģĘä»ÆѧŠŌÖŹ£¬Ź×ĻČ×öĮĖŅų¾µ·“Ó¦.
£Ø1£©ŌŚ¼×Ėį½ųŠŠŅų¾µ·“Ó¦Ē°£¬±ŲŠėŌŚĘäÖŠ¼ÓČėŅ»¶ØĮæµÄ £¬ŅņĪŖ £»
£Ø2£©Š“³ö¼×Ėį½ųŠŠŅų¾µ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø3£©Ä³Ķ¬Ń§×öŅų¾µ·“Ó¦Ź§°Ü£¬ĖūæÉÄܽųŠŠµÄ“ķĪó²Ł×÷ÓŠ__ ______£ØŠ“×ÖÄø£©£ŗ
| A£®ÓĆ½ą¾»µÄŹŌ¹Ü£» | B£®ŌŚÅضČĪŖ2%µÄNH3”¤H 2OÖŠµĪČėÉŌ¹żĮæµÄÅضČĪŖ2%µÄĻõĖįŅų£» 2OÖŠµĪČėÉŌ¹żĮæµÄÅضČĪŖ2%µÄĻõĖįŅų£» |
| C£®ÓĆĮŁŹ±ÅäÖĘŗƵÄŅų°±ČÜŅŗ£» | D£®ŌŚŅų°±ČÜŅŗĄļ¼ÓČėÉŌ¹żĮæµÄ¼×Ėį£» |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź½Ī÷Ź”ø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗŹµŃéĢā
£Ø9·Ö£¬ĆææÕ1·Ö£©Ä³æĪĶā»ī¶ÆŠ”×é¶Ō¼×Ėį½ųŠŠĮĖČēĻĀµÄŹµŃ飬ŅŌŃéÖ¤Ęäŗ¬ÓŠČ©»ł£¬²¢æ¼²ģĘä»ÆѧŠŌÖŹ£¬Ź×ĻČ×öĮĖŅų¾µ·“Ó¦.
£Ø1£©ŌŚ¼×Ėį½ųŠŠŅų¾µ·“Ó¦Ē°£¬±ŲŠėŌŚĘäÖŠ¼ÓČėŅ»¶ØĮæµÄ £¬ŅņĪŖ £»
£Ø2£©Š“³ö¼×Ėį½ųŠŠŅų¾µ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø3£©Ä³Ķ¬Ń§×öŅų¾µ·“Ó¦Ź§°Ü£¬ĖūæÉÄܽųŠŠµÄ“ķĪó²Ł×÷ÓŠ__ ______£ØŠ“×ÖÄø£©£ŗ
A£®ÓĆ½ą¾»µÄŹŌ¹Ü£»B£®ŌŚÅضČĪŖ2%µÄNH3”¤H2OÖŠµĪČėÉŌ¹żĮæµÄÅضČĪŖ2%µÄĻõĖįŅų£»
C£®ÓĆĮŁŹ±ÅäÖĘŗƵÄŅų°±ČÜŅŗ£»D£®ŌŚŅų°±ČÜŅŗĄļ¼ÓČėÉŌ¹żĮæµÄ¼×Ėį£»
E£®·“Ó¦¹ż³ĢÖŠ£¬Ć»ÓŠÕńµ“ŹŌ¹Ü.
Č»ŗó£¬Ķ¬Ń§ĆĒ¶Ō¼×ĖįÓė¼×“¼½ųŠŠĮĖõ„»Æ·“Ó¦µÄŃŠ¾æ£ŗ
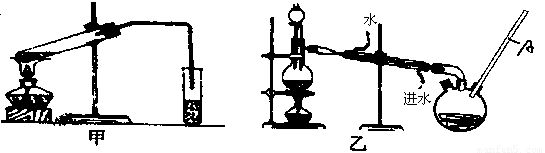
£Ø4£©ŅŅ×°ÖĆÖŠ³¤µ¼¹ÜAµÄ×÷ÓĆŹĒ £»
£Ø5£©Ń”Ōń¼××°ÖĆ»¹ŹĒŅŅ×°ÖĆŗĆ£æ________£¬ŌŅņŹĒ £»
£Ø6£©ŹµŃé¹ż³ĢÖŠŃ”ÓƵÄŅ©Ę·¼°ŹŌ¼ĮÓŠ£ŗÅØH2SO4”¢¼×“¼”¢¼×Ėį»¹ÓŠ________”¢________Į½ÖÖ±Ų±øÓĆĘ·.
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com