
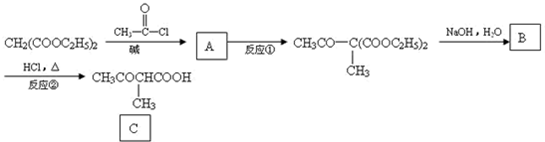
| ��D |
| �� |
| ��NaOH��H2O |
| ��HCl���� |
 COOH��
COOH�� ��
�� ��B���Ȼ��ⷴӦ����C���ٽ�����ʵĽṹ���ʽ��
��B���Ȼ��ⷴӦ����C���ٽ�����ʵĽṹ���ʽ�� ��
�� ��B���Ȼ��ⷴӦ����C��
��B���Ȼ��ⷴӦ����C�� ��
�� ��
�� ��
�� ��
�� ��
�� ��
��| ��D |
| �� |
| ��NaOH��H2O |
| ��HCl���� |
 ��CH2��COOC2H5��2��D����ȡ����Ӧ���ٺ��������Ƶ�ˮ��Һ����ȡ����Ӧ������ٺ��Ȼ��ⷢ��ȡ����Ӧ����
��CH2��COOC2H5��2��D����ȡ����Ӧ���ٺ��������Ƶ�ˮ��Һ����ȡ����Ӧ������ٺ��Ȼ��ⷢ��ȡ����Ӧ���� ����HCl����ȡ����Ӧ��������
����HCl����ȡ����Ӧ�������� ����NaOHˮ��Һ����ȡ����Ӧ��������
����NaOHˮ��Һ����ȡ����Ӧ�������� �����������Ϣ֪��D�Ľṹ��ʽΪClCH2CH2CH2CH2Cl��
�����������Ϣ֪��D�Ľṹ��ʽΪClCH2CH2CH2CH2Cl��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.5NA���������ӵ����ʵ���Ϊ1mol |
| B����״���£�22.4L�����е�ԭ����ĿΪ12NA |
| C��20g D2O�к��еĵ�����Ϊ10NA |
| D��5.6g��������ϡ���ᷴӦʧȥ������Ϊ0.3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������| a | b | c | |
| A | Al | AlCl3 | Al��OH��3 |
| B | Al2O3 | AlO2- | Al��OH��3 |
| C | Na | Na2O | NaOH |
| D | Si | SiO2 | H2SiO3 |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2NO+O2=2NO2 |
| B��N2+O2=2NO |
| C��N2+3H2=2NH3 |
| D��3NO2+H2O=2HNO3+NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���Իش�
���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| X | ||
| M |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com