
�±��У��Գ��������ȷ�Լ������Ƿ���������ϵ���ж϶���ȷ���ǣ� ��
| ѡ�� | ������ | ������ | �ж� |
| A | ��ҵ����������ˮ����SO3 | SO3����ˮ��Ӧ | ��ԣ���ԣ��� |
| B | Cl2��SO2��Ϻ������Ư��ֽ�� | Cl2��SO2���нϺõ�Ư������ | ��ԣ�������� |
| C | �����ƾ���ǿ��ԭ�� | ��ѹ�ƵƷ�������ǿ�Ļƹ� | �������ԣ��� |
| D | ʯī���������صĵ缫 | ʯī�Ļ�ѧ�����ȶ��ҵ����Ժ� | ��ԣ���ԣ��� |
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±��У��Գ��������ȷ�Լ������Ƿ���������ϵ���ж϶���ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
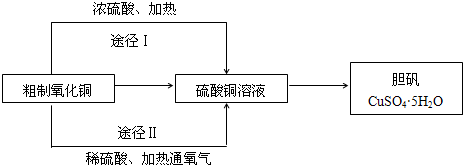
| ||
| ||
| ѡ�� | ������ | ������ | �ж� |
| A | ��ͭ���ҹ�ʹ������ĺϽ� | �Ͻ���ָ���ֽ����ۺϳɵĽ������� | �������ԣ��� |
| B | ����ͭʱ����ͭ�������� | ͭ�������õĵ����� | ��ԣ���ԣ��� |
| C | ͭпԭ����У�ͭ������ | CuO��Cu2O���Ǻ�ɫ���� | ������������ |
| D | CuO����ˮ��Ӧ����Cu��OH��2 | ���������ﶼ����ˮ��Ӧ������Ӧ�Ľ����������� | ��ԣ���ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ʯ���� | ��ͭ�� | ��ͭ�� | ��ͭ�� | ��ȸʯ |
| ��Ҫ�ɷ� | CuFeS2 | Cu5FeS4 | Cu2S | CuCO3?Cu��OH��2 |
| ||
| ѡ�� | ������ | ������ | �ж� |
| A | ͭ�̵����ɷ��Ǽ���ͭ | ����ϡ�����ͭ�������ͭ�� | ��ԣ���ԣ��� |
| B | ͭ�����γ����ܵ�����Ĥ | ͭ��������ʢ��Ũ���� | ��ԣ���ԣ��� |
| C | ����ͭ���� | ����ͭ���ϵ������ڳ�ʪ�����в������� | ��ԣ���ԣ��� |
| D | ��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 | ����ͭ��Һ��������Ӿ�ص������� | �������ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2011?����һģ���±��У��Գ��������ȷ�Լ����������ϵ���жϣ���ȫ��ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ������ | ������ | �ж� |
| A | ����������ò�Ҫʢ�����Ի���Խ�ǿ��Һ��ʳ�� | ��ΪAl��Al2O3�ȿ������ᷴӦ���ֿ�����Ӧ | ��ԣ���ԣ��� |
| B | ��������ʹʪ�����ɫ������ɫ������ʹ�������ɫ������ɫ | ��Ϊ�����ʹ�������н�ǿ�������� | ��ԣ���ԣ��� |
| C | ˫��ˮ�����������ӡ�����ͭ��������ɱ�������� | ��Ϊ˫��ˮ�����������ӡ�����ͭ�����н�ǿ������ | ��ԣ���ԣ��� |
| D | �������ƳɵIJ۳������ܷ�����Ũ�����Ũ���� | ��Ϊ������������Ũ�����Ũ���ᷴӦ | �������ԣ��� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com