
øł¾ŻĻĀĮŠæņĶ¼¹ŲĻµĢīæÕ£¬ŅŃÖŖ·“Ó¦¢Ł”¢¢ŪŹĒ¹¤ŅµÉś²śÖŠµÄÖŲŅŖ·“Ó¦£¬D”¢E³£ĪĀĻĀĪŖĘųĢ唢X³£ĪĀĻĀĪŖĪŽÉ«ŅŗĢ壬HÓėEĻą¶Ō·Ö×ÓÖŹĮæÖ®¼äµÄ¹ŲĻµĪŖ£ŗMr(H) £Mr(E) =34£¬ÓÖÖŖCŃęÉ«·“Ó¦»šŃę³Ź»ĘÉ«”£
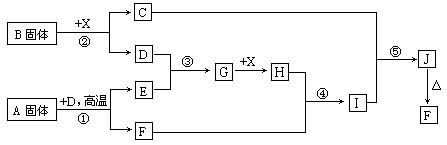
(1) ·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½£ŗ__________________________________£»
²śĪļCµÄµē×ÓŹ½£ŗ_______________________”£
(1)»ÆŗĻĪļAÖŠĖł°üŗ¬µÄ»Æѧ¼üÓŠ£ŗ_______________________”£
(2)·“Ó¦¢ÜµÄĄė×Ó·½³ĢŹ½£ŗ_______________________________”£
·“Ó¦¢ŻµÄ»Æѧ·½³ĢŹ½£ŗ__________________________________”£
(3)ŅŃÖŖĆæÉś³É16g E£¬·Å³ö106.5 kJČČĮ棬Ōņ·“Ó¦¢ŁµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ
_________________________________________________________ ӣ
“š°ø½āĪö£ŗ£ØÖŠÄŃ£©×„×”·“Ó¦¢Ł”¢¢ŪŹĒ¹¤ŅµÉś²śÖŠµÄÖŲŅŖ·“Ó¦ÕāøöĶ»ĘĘæŚ£¬ŌŁ½įŗĻAŹĒ¹ĢĢ壬µĆ³ö£ŗ·“Ó¦¢ŁŹĒģŃÉÕFeS2ÖĘSO2£¬XŹĒĪŽÉ«ŅŗĢ壬ӦĪŖĖ®£¬¢ŪŹĒÖĘSO3£¬¢ŚŹĒ¹ĢĢåBÓėĖ®×÷ÓĆ²śÉśO2£¬Ōņ¹ĢĢåBĪŖ¹żŃõ»ÆÄĘ£¬ĘäĖū¾ĶŗĆ½āĮĖ”£²Ī漓š°ø¹éÄÉČēĻĀ£ŗ
””””(1) 2Na2O2+2H2O===4NaOH+O2”ü£Ø2·Ö£©![]() £Ø2·Ö£©
£Ø2·Ö£©
(1)Ąė×Ó¼ü”¢·Ē¼«ŠŌ¼ü £Ø3·Ö£¬“š³ö1øöÖ»øų1·Ö£©
(2)Fe2O3 + 6H+ = 2Fe3+ + 3H2O£Ø2·Ö£©
Fe2(SO4)3 + 6NaOH = 2Fe(OH)3”ż+3Na2SO4£Ø2·Ö£©
””””(3)FeS2(s) + 11/4 O2(g) = 1/2 Fe2O3(s)+ 2SO2(g)£»”÷H=£852kJ/mol£Ø4·Ö£©
¼¼ÄÜæÕ¼ä£ŗ±¾Ģā½āĢāĶ»ĘĘæŚŹĒ”°ÖŲŅŖ¹¤ŅµÉś²ś·“Ó¦”±”£ÖŠŃ§ÖŲŅŖ¹¤ŅµÉś²ś·“Ó¦ÓŠ£ŗ1£®ģŃÉÕŹÆ»ŅŹÆ 2£®ģŃÉÕ»ĘĢśæó 3£®¶žŃõ»ÆĮņµÄ“ß»ÆŃõ»Æ 4£®°±µÄ“ß»ÆŃõ»Æ 5£®ŗĻ³É°± ””6£®µē½ā±„ŗĶŹ³ŃĪĖ® 7£®¹¤ŅµÖĘŃĪĖį 8£®øßĀÆĮ¶Ģś 9£®¹¤ŅµÖĘČ”ĘÆ·Ū¾« 10£®¹¤ŅµÖĘĖ®ĆŗĘų 11£®¹čĖįŃĪ¹¤ŅµµČ”£
Ó¦ŹŌ²ßĀŌ£ŗÓŠŅ»ĄąæņĶ¼ĶʶĻĢā£¬æ¼²é֊ѧĖłŃ§µÄ¹¤ŅµÉś²śŌĄķ£¬²¢½įŗĻŌŖĖŲ»ÆŗĻĪļµÄÖŖŹ¶£¬×ŪŗĻ漲鹤ŅµÉś²śÖŠ³£¼ū»ÆŗĻĪļµÄŠŌÖŹ£¬ÓŠŅ»¶ØµÄ×ŪŗĻŠŌ”£ĢāÄæÓŠŅ»¶ØÄŃ¶Č£¬³żÓŠŌśŹµµÄ»ł±¾¹¦Ķā£¬»¹ŅŖÓŠŅ»¶ØĮé»īŌĖÓĆÖŖŹ¶µÄÄÜĮ¦£¬½ĻĒæµÄĶĘĄķÄÜĮ¦”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
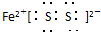
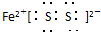
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
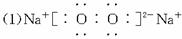
£Ø1£©Š“³ö»ÆŗĻĪļBµÄµē×ÓŹ½_____________________”£
£Ø2£©·“Ó¦¢ÜµÄĄė×Ó·½³ĢŹ½ĪŖ_____________________”£
·“Ó¦¢ŻµÄ»Æѧ·½³ĢŹ½ĪŖ___________________________________________________”£
£Ø3£©ŅŃÖŖĆæÉś³É16 g E£¬·Å³ö106.5 kJµÄČČĮ棬Ōņ·“Ó¦¢ŁµÄČČ»Æѧ·½³ĢŹ½ĪŖ_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
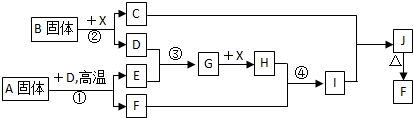
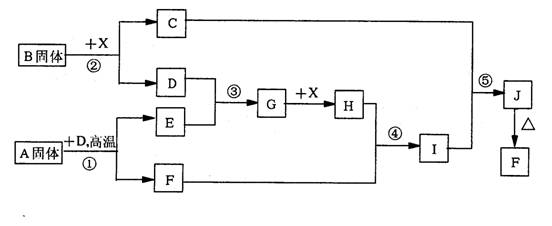
£Ø10·Ö£©øł¾ŻĻĀĮŠæņĶ¼¹ŲĻµĢīæÕ”£ŅŃÖŖ³£ĪĀĻĀXĪŖĪŽÉ«ŅŗĢ壬D”¢E¾łĪŖĪŽÉ«ĘųĢ壬
GŹĒŅ»ÖÖÖŲŅŖµÄ¹¤Ņµ²śĘ·”£CµÄŃęÉ«·“Ó¦ĪŖ»ĘÉ«£¬E”¢FµÄĻą¶Ō·Ö×ÓĮæÖ®¼äµÄ¹ŲĻµĪŖ
M£ØF£©=M£ØE£©+16”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AÖŠ»Æѧ¼üµÄĄąŠĶĪŖ £¬Š“³öBµÄŅ»øöÓĆĶ¾ ”£
£Ø2£©½«¹ĢĢåA¼ÓČėFeCl3ČÜŅŗÖŠµÄÖ÷ŅŖĻÖĻóŹĒ ”£
£Ø3£©Š“³öĘųĢåBµÄĖ®ČÜŅŗÓėCuSO4ČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
£Ø4£©Š“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ ”£
£Ø5£©¾²ā¶Ø£¬·“Ó¦¢ÜÖŠĆæÉś³É1.0gX£¬·Å³ö3.2KJµÄČČĮæ£Ø³£ĪĀĻĀ£©”£ŹŌŠ“³ö·“Ó¦¢Ü
µÄČČ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ½Ī÷Ź”¼Ŗ°²ŹŠøßČżÉĻѧʌÖܲā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(12·Ö)øł¾ŻĻĀĮŠæņĶ¼¹ŲĻµĢīæÕ”£ŅŃÖŖ·“Ó¦¢Ł”¢¢ŪŹĒ¹¤ŅµÉś²śÖŠµÄÖŲŅŖ·“Ó¦£¬D”¢E³£ĪĀĻĀĪŖĘųĢ唢X³£ĪĀĻĀĪŖĪåÉ«ŅŗĢ壬HÓėEĻą¶Ō·Ö×ÓÖŹĮæÖ®¼äµÄ¹ŲĻµĪŖ£ŗMr(H)-Mr(E)=34£¬ÓÖÖŖCµÄŃęÉ«·“Ó¦³Ź»ĘÉ«”£
(1)»ÆŗĻĪļBÖŠĖł°üŗ¬µÄ»Æѧ¼üÓŠ___________________
(2)·“Ó¦¢ÜµÄĄė×Ó·½³ĢŹ½£ŗ________________________________
·“Ó¦¢ŻµÄ»Æѧ·½³ĢŹ½£ŗ________________________________
(3)ŅŃÖŖĆæÉś³É16gE£¬·Å³ö106.5kJČČĮ棬Ōņ·“Ó¦¢ŁµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ
______________________________________
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com