
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�~���ڱ��е�λ�ã��ش��������⣺

(1)�ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ����_______��
(2)�ڵ����������ķ���ʽΪ____��
(3)�١��ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������Ҫ���һ�ֻ�����ĵ���ʽ_____��
(4)W�ǵ����������ͬ�����Ԫ�ء��ݴ��Ʋ�W�����ܾ��е�������___
A.��������ϼ�Ϊ+6 B.��̬�⻯���H2S�ȶ�
C.����������Ӧˮ��������Ա������� D.�����ڳ����¿�����������
(5)��֪XΪ�ڢ�A��Ԫ��(��һ����������)����ԭ������Ϊa��Y��Xλ��ͬһ���ڣ���Ϊ�ڢ�A��Ԫ�أ���Y��ԭ������b��a���п��ܵĹ�ϵʽΪ____��
���𰸡��������ڵڢ�A�� CO2 NaOH��![]() ��Na2O2��
��Na2O2��![]() BD b=a+1��b=a+11
BD b=a+1��b=a+11
��������
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
(1)�ؿ��к������ڵڶ�λ��Ԫ��ΪSi��
(2)�ڱ�ʾCԪ�أ�����Ԫ������ϼ۵���ԭ����������������ԭ������������
(3)��H��O��Na�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ�������NaOH��Na2O2�ȣ�
(4)W�ǵ����������ͬ����Ԫ�أ�����OԪ�أ���WΪSeԪ�أ�����Ԫ�������ɷ����жϣ�
(5)����Ԫ�����ڱ���λ����ԭ��������ϵ�������
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
(1)�ؿ��к������ڵڶ�λ��Ԫ��ΪSi��Siԭ�Ӻ�������Ų�Ϊ2��8��4������Si����Ԫ�����ڱ��е������ڵڢ�A�壻
(2)�ڱ�ʾCԪ�أ�Cԭ���������4�����ӣ����������������ķ���ʽΪCO2��
(3)��H��O��Na�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ�������NaOH��Na2O2�ȣ�����NaOH�ĵ���ʽΪ��![]() ��Na2O2�ĵ���ʽΪ��
��Na2O2�ĵ���ʽΪ��![]() ��
��
(4)W�ǵ����������ͬ����Ԫ�أ�����OԪ�أ���WΪSeԪ�ء�
A. Seԭ���������6�����ӣ���������������ϼ�Ϊ+6��A��ȷ��
B. Ԫ�صķǽ�����Խǿ������Ӧ�ļ��⻯����ȶ��Ծ�Խǿ������Ԫ�صķǽ����ԣ�S>Se��������̬�⻯���ȶ��ԣ�H2S>H2Se��B����
C. ͬһ�����Ԫ��ԭ������Խ��Ԫ�صķǽ�����Խǿ��������������Ӧˮ���������Խǿ�����ڷǽ�����S>Se������H2SeO4<H2SO4��C��ȷ��
D. Ԫ�صķǽ�����Խǿ���䵥��Խ�������������ϣ�����Ԫ�صķǽ����ԣ�S>Se��S��H2��Ӧ���ڼ��������½��У���Se������H2��ӦҪ���¶Ȼ���ߣ��ڳ����²�������H2���ϣ�D����
�ʺ���ѡ����BD��
(5)Xԭ������Ϊa��Yԭ������Ϊb����Xλ�ڵڶ����ڵڢ�A��Ԫ�أ���Xλ�ڵ������ڵ�IIA�壬����ͬһ���ڵ�IIIA��Ԫ��Yԭ������Ϊb=a+1����Xλ�ڵ������ڵڢ�A��Ԫ�أ����ڵ�IIA�塢��IIIA��֮��������7�������1����VIII��Ԫ�أ���10���У�������ͬһ���ڵ�IIIA��Ԫ��Yԭ������Ϊb=a+10+1=a+11��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���̷�(FeSO4��7H2O)������ȱ����ƶѪҩƷ����Ҫ�ɷ֡��������������������������������������ʣ�Ϊԭ�����������̷���һ�ַ�����
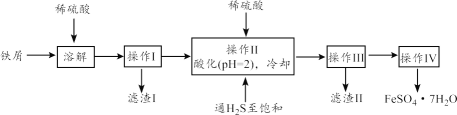
��֪�������±���H2S��Һ��pHԼΪ3.9��SnS������ȫʱ��Һ��pHΪ1.6��FeS��ʼ����ʱ��Һ��pHΪ3.0��������ȫʱ��pHΪ5.5��
��1����ҵ�����ù���ϡ�����ܽ���м����������Ⱦ�����壬�䷴Ӧ���ӷ���ʽ��_____��
��2������II�У�ͨ�����������͵�Ŀ����_________������Һ���������ữ��pH=2��Ŀ����_____��
��3�������£�Ksp[Fe(OH)3]=4.0��10-38����Fe3+��ˮ�ⳣ��Ϊ_____��
��4������IV�õ����̷�������������ˮϴ�ӣ���Ŀ���ǣ�����ȥ������渽�ŵ���������ʣ���_____��
��5�����õ��̷�(FeSO4��7H2O)��������O2��������������Fex(OH)y(SO4)z��wH2O���ֲⶨ����ɣ��������£��������ʲ����뷴Ӧ����ÿ����Ӧ����ȫ���У���
��ȡ���ȷݹ�����Ʒ��
��һ����Ʒ������ϡ�����ܽ����250mL����ƿ�ж��ݣ���ȡ25.00mL������Һ����ƿ�У���0.03mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һ���Ϊ20.00mL���ζ�ʱ��Ӧ�����ӷ���ʽΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O�����ζ�����Һ������pH����Fe3+�����ˡ�ϴ�ӡ���ɲ����գ����յõ�0.32gFe2O3��(Fe2O3ʽ��160)
�ڶ�����Ʒ������ϡ�����ܽ���ټ������BaCl2��Һ�������ˡ�ϴ�ӡ���ɣ���9.32gBaSO4������(BaSO4ʽ��233)
��������Ʒ�����³�����պ��ռ���4.05gH2O�����Ƶ�������(Fex(OH)y(SO4)z��wH2O)�Ļ�ѧʽ��д��������̣�x��y��z��wΪ��������ȣ�______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
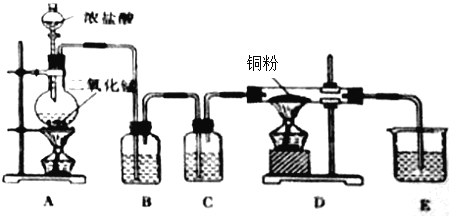
����Ŀ����һ����![]() ��Ũ������ȡ�������������������������ͭ�۷�Ӧ��ȡ��������ˮ
��Ũ������ȡ�������������������������ͭ�۷�Ӧ��ȡ��������ˮ![]() ��װ������ͼ��ʾ��
��װ������ͼ��ʾ��
![]()
�ش��������⣺
��1��д����A�з�����Ӧ�Ļ�ѧ����ʽΪ��_______________________________________________
��2��B��ѡ�õ��Լ���______________����������________________________��C��ѡ�õ��Լ���______________����������________________________��E��ѡ�õ��Լ���_____________����������________________________��
��3��D�з�Ӧ�Ļ�ѧ����ʽ��________________________________________________
������ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�![]() �Ĺ�������ˮ������
�Ĺ�������ˮ������![]() �ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ�������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⡣
�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ�������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⡣
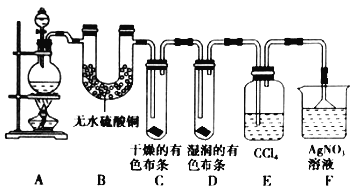
��4�����ú���![]() ��Ũ������������
��Ũ������������![]() ��Ӧ��
��Ӧ��![]() ���Ƶõ�
���Ƶõ�![]() �������״���£�����С��
�������״���£�����С��![]() ��ԭ����____________________________________________________��
��ԭ����____________________________________________________��
��5����װ��B��������_______________________________________________��������_______________________________________________��
��װ��C��D���ֵIJ�ͬ����˵����������_______________________________________________��
��װ��E��������_______________________________________________��
��6����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ�����ȷ������ͨ��![]() ��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��
��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��![]() ��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�һ��װ�á�����Ϊ��װ��Ӧ����_________��_________֮�䣨��װ����ĸ��ţ���װ����Ӧ����____________________________________________��
��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�һ��װ�á�����Ϊ��װ��Ӧ����_________��_________֮�䣨��װ����ĸ��ţ���װ����Ӧ����____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����������������ȷ���ǣ� ��
A.һ���¶Ⱥ�ѹǿ�£�������̬��������Ĵ�С���ɹ�������ķ��Ӵ�С����
B.һ���¶Ⱥ�ѹǿ�£�������̬��������Ĵ�С���ɹ�������ķ���������
C.��ͬ�����壬�������ͬ�������������ķ�����Ҳ��ͬ
D.����Ħ�����ָ1mol�κ�������ռ�����ԼΪ22.4L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ӣ����ѧ��ϣ�����֣������Ҷ��������Һ�м����ʵ��ĵ�������(�������)���ڹ���ʱ��ʹˮ�ֽ���ͷ��������Ӷ�֤���������ͷ���CO2��ԭ�Dz�ͬ�Ĺ��̣����Թ�����õ��о�������ϸ�����档�÷�Ӧ����ʽΪ4Fe3++2H2O![]() 4Fe2++4H++O2���������й�ϣ����Ӧ˵��������ǣ� ��
4Fe2++4H++O2���������й�ϣ����Ӧ˵��������ǣ� ��
A. ˮ�ڹⷴӦ��������͵��ӹ���˫������
B. ��Ӧ����ҺpH��С
C. ���ɱ�״����11.2 L O2ʱ��Fe3+�õ�����Ϊ2NA
D. Ҷ���屾��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(K2FeO4)��һ��������ˮ��������25�棬��ˮ��Һ�м�����ı���Һ��pHʱ���������ӵ����ʵ���������(X)��pH�ı仯��ͼ��ʾ[��֪��(X)= ]������˵����ȷ���ǣ� ��
]������˵����ȷ���ǣ� ��
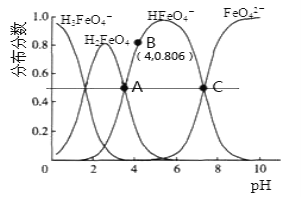
A.K2FeO4��H2FeO4������ǿ�����
B.25�棬H2FeO4+H+![]() H3FeO4+��ƽ�ⳣ��K>100
H3FeO4+��ƽ�ⳣ��K>100
C.��B�����ݿ�֪��H2FeO4�ĵ�һ�����볣��Ka1=4.15��10-4
D.A��C�����Ӧ��Һ��ˮ�ĵ���̶����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������壨FeSO4��7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����������ò�Ѫ���Ƿ���ʡ�ʵ�鲽�����£�

��ش��������⣺
��1��������ٵ���Һ�еμ�KSCN��Һ���Ϊ��ɫ�������Һ�к���______�������ӷ��ţ���
��2���������з�Ӧ�����ӷ���ʽ��__________________________________��
��3���������з�Ӧ�����ӷ���ʽ��__________________________________��
��4����������һϵ�д����IJ������裺���ˡ�______�����ա�_______��������
��5������ʵ���е���ĺ��Բ��ƣ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ______g�����ú�a�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��״������LiNi0.8Co0.1Mn0.1O2(NCM811)��Ϊ��һ������ӵ�ص������ı��㷺��ע�������о�������TiO2�γɵı����������߸ò��ϵ�����Ч�����ԡ��ش��������⣺
��1��Li��Ԫ�����ڱ��е�λ��Ϊ___����̬Ni�ĵ����Ų�ʽΪ___����̬Co3+��__��δ�ɶԵ��ӡ�
��2���Ʊ�NCM811�Ĺ����У������Li2CO3���ƻ����ϵĽ��棬CO32-�Ŀռ乹����___������Cԭ�ӵ��ӻ���ʽΪ___��
��3���õ�س�ʼ�������У�����C2H4�����������C2H4�����С�![]() ����
����![]() ����Ŀ֮��Ϊ__��
����Ŀ֮��Ϊ__��
��4��TiO2�ľ�����![]() =
=![]() =
=![]() =90o����ͼ��ʾ��
=90o����ͼ��ʾ��
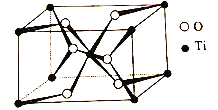
TiO2������Oԭ�ӵ���λ����__���侧������Ϊ��a=b=459pm��c=295pm���þ�����ܶ�Ϊ__g/cm3���г�����ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��Ԫ�����ڱ���1��20���Ҳ�ͬ�����Ԫ����ɵĵ��ʼ�������֮���ת����ϵ�������е�ˮ����ȥ��������AΪ����ɫ���嵥�ʣ�D��Ư���ԣ���ʵ�����г��ù���B����C������ȡ�̼�����ζF��F��G�����Ԫ����ͬ��G��H����������������ͬ��
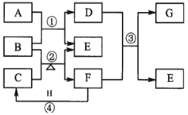
��ش�
(1)����A�����Ԫ�������ڱ��е�λ����________��
(2)B�Ļ�ѧʽΪ________��F�ĵ���ʽΪ________��C��������ѧ��������_______��
(3)д����Ӧ�ڵĻ�ѧ����ʽ_____________��
(4)��Ӧ����F������H�ڿ���������ʱ��ʵ������Ϊ__________д����Fһ�־�����;_______________��
(5)�����Ư�����õ�D��Һ�м���H��Ũ��Һ��A���ɣ��䷴Ӧ�����ӷ���ʽΪ______________��
(6)��Ԫ��(As)������ijԪ�ش���ͬһ���塣����Ǧ������ɱ�������֪��
��������Ǧ�У��鴦�����̬��Ǧ�����ȶ���̬��
������Ǧ���������Ӧ���Σ�1mol����������к���8molԭ��.
������������Ļ�ѧʽΪ___________����Ǧ�Ļ�ѧʽΪ________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com