
��һ�����ľ���A�������������ȵ�200��ʱ��Aȫ���ֽ�Ϊ�����ʵ������������塣�������������������ת����ϵ����ͼ��ʾ��F��J����ѧ��ѧ�г��������ֵ��ʡ�HΪ����ɫ���塣ͼ�в��ַ�Ӧ������������û���г����밴Ҫ����գ�
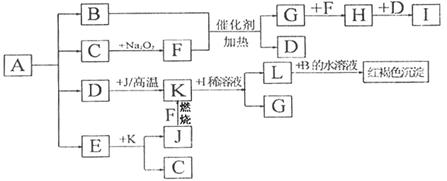
��1������F�Ļ�ѧʽ
��2��д��B��F��Ӧ�Ļ�ѧ����ʽ ��L��B��ˮ��Һ��Ӧ�����ӷ���ʽ ��
��3��д��K��I��ϡ��Һ��Ӧ�����ӷ���ʽ
��4��A�Ļ�ѧʽΪ
��5����ҵ��������������ϡ�������������Ϊԭ�����Ʊ�ij��Ч��ˮ��Fe(OH)SO4,��Ӧ��G���ɣ��÷�Ӧ�Ļ�ѧ����ʽ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾװ��Ϊ��ֱ����������µ��CuSO4 ��Һͼ������A��BΪʯī�缫��
a��bΪ��Դ������������ͨ��Դ��ͨ��һ��ʱ���B�缫ȡ��ϴ�ɾ�����������������������3��2g����
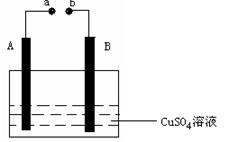 ��1�� aΪ��Դ�� ����
��1�� aΪ��Դ�� ����
bΪ��Դ�� ��
��2�� д���缫��Ӧ����ʽ��
A ��
B ��
��3��A�缫�����������Ϊ L���ڱ�״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڵ������Һ��������ȷ����
A�������£�pH��7��NH4Cl�백ˮ�Ļ����Һ������Ũ�ȴ�С˳��Ϊ��
c (Cl��) > c (NH4+) > c (H��)> c (OH��)
B����pH��4�Ĵ�����Һϡ�ͺ���Һ���������ӵ�Ũ�Ⱦ�����
C���к�pH���������ͬ������ʹ�����Һ������NaOH�����ʵ�����ͬ
D�������£�ͬŨ�ȵ�Na2S��NaHS��Һ��ȣ�Na2S��Һ��pH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ���� (����)
A��Ũ�����м���������۲����ȣ�Fe��3NO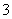 ��6H��=Fe3����3NO2����3H2O
��6H��=Fe3����3NO2����3H2O
B��Ca(HCO3)2��Һ�����NaOH��Һ��Ӧ HCO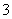 ��OH����Ca2��===CaCO3����H2O
��OH����Ca2��===CaCO3����H2O
C���������������������Һ��Fe(OH)3��3H��===Fe3����3H2O
D������������ʵ���Ũ�ȵ�����������Һ��̼�������Һ��ϣ�
Ba2����2OH����NH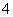 ��HCO
��HCO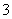 ===BaCO3����NH3��H2O��H2O
===BaCO3����NH3��H2O��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ����Fe��Fe2O3�Ļ����Ͷ��250 mL 2 mol/L�������У���Ӧ������1.12 L NO(��״����)������Ӧ�����Һ�м���1 mol/L��NaOH��Һ����������ȫʱ����NaOH��Һ�����������(����)
A��450 mL B��500 mL C��400 mL D������ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ� ��
A��ͬ����ǽ���Ԫ�صļ������ӵĻ�ԭ��Խǿ����Ԫ�طǽ�����Խǿ
B����A�����A��Ԫ�ؼ���γɹ��ۻ���������ӻ�����
C��Ԫ��ԭ�ӵ���������������Ԫ�ص�����ϼ�
D��ȫ���ɷǽ���Ԫ����ɵĻ�������ֻ�����ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Cl2�Ƿ�֯��ҵ�г��õ�Ư����Na2S2O3����Ư�ײ�ƥ��ġ����ȼ����� ���ȷ�ӦΪ
S2O32����Cl2��H2O��SO42����Cl����H����δ��ƽ�������жԸ÷�Ӧ��˵������ȷ���ǣ� ��
A����Ӧ����Ԫ�ط�����������Ӧ B�����ȷ�Ӧ�����Һ������
C�����ݸ÷�Ӧ���жϻ�ԭ�ԣ�S2O32����Cl�� D����Ӧ��ÿ��ȥ1mol Cl2������1 mol SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾij��̬����A���仯����֮���ת����ϵ(ijЩ����ͷ�Ӧ��������ȥ)��������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϡ�
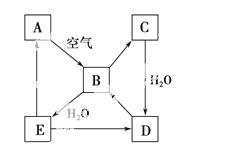
(1)д��A�ڼ�����������H2��Ӧ�Ļ�ѧ����ʽ��
_________________________________________________________________��
(2)д��E��A���⻯�ﷴӦ����A�Ļ�ѧ����ʽ��
_________________________________________________________________��
(3)д��һ����D����B�Ļ�ѧ����ʽ��
________________________________________________________________��
(4)��5 mL 0.10 mol·L��1��E��Һ��10 mL 0.10 mol·L��1��NaOH��Һ��ϡ�
��д����Ӧ�����ӷ���ʽ�� _________________________________________��
�ڷ�Ӧ����Һ��pH________7(����ڡ���С�ڡ����ڡ�)��������______________________________________________________________ ___��
___��
�ۼ��ȷ�Ӧ�����Һ����pH________(��������䡱��С��)��������_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A��Ԫ�ص����ԭ��������С����������Ԫ�ؾ���ͬλ��
B������ͬ��������ԭ�ӻ�����һ������ͬ��Ԫ��
C��K����Ar������ͬ�ĵ��Ӳ�ṹ��������K����Arǿ
D���������ӵĵ��Ӳ���һ���Ƚ���ԭ�ӵĵ��Ӳ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com