

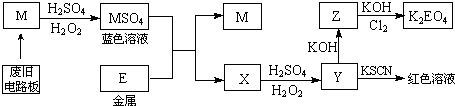
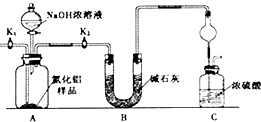
���� ��1�����Ļ��ϼ�һ��Ϊ���ۣ���̼�ķǽ���С����ķǽ����ԣ����Sһ����ʾ���ۣ�
��2��װ��A�й��������ڶ�������Ϊ����������������������ͨ��Ũ��������װ��C�е�̼Ԫ������Ϊ������̼����Ԫ������Ϊ����������������ͨ������������Һ�����������������գ�������̼ͨ��Eװ������̼�ᱵ���������û��˫��ˮ����������������Ҳ��������������Ӧ���ɳ�����ʹ̼�İٷֺ���ƫ�ߣ�
��3��������̼ͨ��Eװ������̼�ᱵ������
��4����E�г���������Ϊbg����̼�ᱵ������������̼Ԫ���غ��֪̼�ᱵ�����ʵ����������̼�����ʵ�����ȣ��ݴ˼���������̼������������������̼������������
��5������װ��D����Һ���Ƿ��������������֤�����Ƿ�����Ԫ�أ������ǣ�ȡ����Dƿ��Һ���Թ��У��μ������ữ��BaCl2��Һ�������ְ�ɫ������˵�������к�����Ԫ�أ�
��6��ʵ�������Ӧע��İ�ȫ�����У�Ũ�����и�ʴ�ԣ�ʹ��ʱҪС�ģ�����ʱҪʹ��ͨ�ܾ������ȵȣ�
��� �⣺��1�����Ļ��ϼ�һ��Ϊ���ۣ���̼�ķǽ���С����ķǽ����ԣ����Sһ����ʾ���ۣ���ѡA��
�ʴ�Ϊ��A��
��2�����û��˫��ˮ����������������Ҳ��������������Ӧ���ɳ�����ʹ̼�İٷֺ���ƫ�ߣ���D��30% ˫��ˮ������������SO2���壬��Ӧ�����ӷ���ʽΪ��H2O2+SO2=2H++SO42-��
�ʴ�Ϊ��H2O2+SO2=2H++SO42-��ƫ�ߣ�
��3��������̼������������Ӧ����̼�ᱵ��ˮ����Ӧ���ӷ���ʽΪCO2+Ba2++2OH-=BaCO3��+H2O����Ӧ����Ϊ���ɰ�ɫ������
�ʴ�Ϊ���а�ɫ�������ɣ�CO2+Ba2++2OH-=BaCO3��+H2O��
��4����E�еõ�bg̼�ᱵ������̼Ԫ���غ��֪̼�ᱵ�����ʵ����������̼�����ʵ�����ȣ���������̼������Ϊ$\frac{bg}{197g/mol}$��12g/mol=$\frac{12b}{197}$g��������̼����������Ϊ$\frac{\frac{12b}{197}g}{ag}$��100%=$\frac{12b}{197a}$��100%��
�ʴ�Ϊ��$\frac{12b}{197a}$��100%��
��5��ȡ����Dƿ��Һ���Թ��У��μ������ữ��BaCl2��Һ�������ְ�ɫ������˵�������к�����Ԫ�أ�
�ʴ�Ϊ��ȡ����Dƿ��Һ���Թ��У��μ������ữ��BaCl2��Һ�������ְ�ɫ������˵�������к�����Ԫ�أ�
��6��ʵ�������Ӧע��İ�ȫ�����У�Ũ�����и�ʴ�ԣ�ʹ��ʱҪС�ģ�����ʱҪʹ��ͨ�ܾ������ȵȣ�
�ʴ�Ϊ��Ũ�����и�ʴ�ԣ�ʹ��ʱҪС�Ļ����ʱҪʹ��ͨ�ܾ������ȵȣ�
���� ���⿼���ʵ�鷽�������װ�õ����⡢ʵ�������������ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ������֪ʶ�������⡢����������������������Ͷ�����̼������������������������ƣ���˸߿��ж�������������ļ���ıȽ϶࣬�������������Ӧ������һ���㣬��������������ʼ��Կ��飮


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| V��M����x10-3mol��L-1��min-1�� | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���¶��£�0.0l mol/L������Һ��pH=4 | |
| B�� | ���¶��£���0.1 mol/L�����0.01 mol/L����ֱ���ȫ�к͵����0.1 mol/L��NaOH��Һ������������������Ϊ1��10 | |
| C�� | ���¶��£�0.2 mol/L������Һ��0.4 mol/L��������Һ�������Ϻ��Һ��pH��4.7 | |
| D�� | ���¶��£�0.2 mol/L������Һ��0.4 mol/L��������Һ�������Ϻ��Һ��pH��4.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2+Cl2�T2HCl | B�� | CuO+2H+�TCu2++H2O | ||
| C�� | H2O+CaO�TCa��OH��2 | D�� | NaOH+HCl�TH2O+NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���³�ѹ�£�14 g��ϩ�Ͷ�ϩ�Ļ�����庬�е�ԭ����Ϊ6NA | |
| B�� | 25�棬pH=1��1 L H2SO4��Һ���е�H+��ĿΪ0.2NA | |
| C�� | 1 mol Fe��һ������HNO3��Ӧ��ת�Ƶĵ�����Ŀһ��Ϊ3NA | |
| D�� | ��״���£�22.4 L NO��O2�Ļ������������ԭ����Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���³�ѹ�£�17 g ND3������������ĿNA | |
| B�� | 25�棬pH=11��Na2CO3��Һ����ˮ�������H+����ĿΪ10-3NA | |
| C�� | �ö��Ե缫���CuSO4��Һ���������0.1mol Cu��OH��2��ʹ��Һ��ԭ�����·��ת�Ƶ��ӵ���ĿΪ0.4NA | |
| D�� | 6g SiO2����������Ϊ0.1NA����ѧ������Ϊ0.4 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| W | X | |
| Y | Z |
| A�� | X��Y��Z����Ԫ�ص�����⻯������ȶ�����Y | |
| B�� | W��Y��Z����Ԫ�ض�Ӧ�������ˮ����һ������ǿ�� | |
| C�� | W��XԪ�ص�����⻯�ﶼ�Ƿǵ���� | |
| D�� | ZԪ�صĵ����ڻ�ѧ��Ӧ��ֻ�ܱ��������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com