
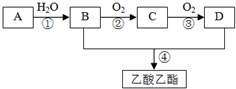
 AŹĒŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·Ö£¬AµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤Ė®Ę½£®ĻÖŅŌAĪŖÖ÷ŅŖŌĮĻŗĻ³ÉŅŅĖįŅŅõ„£¬ĘäŗĻ³ÉĀ·ĻßČēĶ¼ĖłŹ¾£®
AŹĒŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·Ö£¬AµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤Ė®Ę½£®ĻÖŅŌAĪŖÖ÷ŅŖŌĮĻŗĻ³ÉŅŅĖįŅŅõ„£¬ĘäŗĻ³ÉĀ·ĻßČēĶ¼ĖłŹ¾£® CH3COOCH2CH3+H2O£»
CH3COOCH2CH3+H2O£»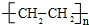 £®
£® ·ÖĪö AŹĒŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·Ż£¬ĖüµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤Ė®Ę½£¬ŌņAŹĒCH2=CH2£¬ŅŅĻ©ŗĶĖ®·¢Éś¼Ó³É·“Ӧɜ³ÉBĪŖCH3CH2OH£¬ŅŅ“¼·¢ÉśŃõ»Æ·“Ӧɜ³ÉCĪŖCH3CHO£¬ŅŅČ©½ųŅ»²½·¢ÉśŃõ»Æ·“Ӧɜ³ÉDĪŖCH3COOH£¬ŅŅĖįÓėŅŅ“¼·¢Éśõ„»Æ·“Ӧɜ³ÉŅŅĖįŅŅõ„£¬¾Ż“Ė½ā“š£®
½ā“š ½ā£ŗAŹĒŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·Ż£¬ĖüµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤Ė®Ę½£¬ŌņAŹĒCH2=CH2£¬ŅŅĻ©ŗĶĖ®·¢Éś¼Ó³É·“Ӧɜ³ÉBĪŖCH3CH2OH£¬ŅŅ“¼·¢ÉśŃõ»Æ·“Ӧɜ³ÉCĪŖCH3CHO£¬ŅŅČ©½ųŅ»²½·¢ÉśŃõ»Æ·“Ӧɜ³ÉDĪŖCH3COOH£¬ŅŅĖįÓėŅŅ“¼·¢Éśõ„»Æ·“Ӧɜ³ÉŅŅĖįŅŅõ„£¬
£Ø1£©BĪŖCH3CH2OH£¬ŗ¬ÓŠ¹ŁÄÜĶÅĪŖōĒ»ł£¬DĪŖCH3COOH£¬ŗ¬ÓŠµÄ¹ŁÄÜĶÅĪŖōČ»ł£¬
¹Ź“š°øĪŖ£ŗōĒ»ł£»ōČ»ł£»
£Ø2£©·“Ó¦¢ŁŹōÓŚ¼Ó³É·“Ó¦£¬·“Ó¦¢ÜŹōÓŚõ„»Æ·“Ó¦»ņČ”“ś·“Ó¦£¬
¹Ź“š°øĪŖ£ŗ¼Ó³É·“Ó¦£»õ„»Æ·“Ó¦»ņČ”“ś·“Ó¦£»
£Ø3£©·“Ó¦¢ŚŹĒŅŅ“¼·¢Éś“ß»ÆŃõ»ÆÉś³ÉŅŅČ©£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗ2CH3CH2OH+O2$”ś_{”÷}^{Cu}$2CH3CHO+2H2O£¬
·“Ó¦¢ÜµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗCH3COOH+CH3CH2OH CH3COOCH2CH3+H2O£¬
CH3COOCH2CH3+H2O£¬
¹Ź“š°øĪŖ£ŗ2CH3CH2OH+O2$”ś_{”÷}^{Cu}$2CH3CHO+2H2O£»CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O£»
CH3COOCH2CH3+H2O£»
£Ø4£©ŅŅĻ©ŌŚŅ»¶ØĢõ¼žĻĀæɾŪŗĻÉś³É¾ŪŅŅĻ©£¬ŗĻ³ÉøĆĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗnCH2=CH2$\stackrel{“߻ƼĮ}{”ś}$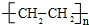 £¬
£¬
¹Ź“š°øĪŖ£ŗnCH2=CH2$\stackrel{“߻ƼĮ}{”ś}$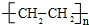 £®
£®
µćĘĄ ±¾Ģāæ¼²éĪŽ»śĪļĶʶĻ£¬Éę¼°Ļ©Ģž”¢“¼”¢Č©”¢ōČĖįµÄŠŌÖŹÓė×Ŗ»Æ£¬±Č½Ļ»ł“”£¬²ąÖŲ¶Ō»ł“”ÖŖŹ¶µÄ¹®¹Ģ


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĘųĢ¬Ēā»ÆĪļµÄĪČ¶ØŠŌ£ŗHX£¾H2Y£¾ZH3 | B£® | ·Ē½šŹō»īĘĆŠŌ£ŗY£¼X£¼Z | ||
| C£® | Ō×Ó°ė¾¶£ŗX£¾Y£¾Z | D£® | Ō×Ó×īĶā²ćµē×ÓŹż£ŗZ£¾Y£¾X |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na2O2ÓėCO2 | B£® | NaÓėO2 | C£® | NaOHÓėCO2 | D£® | NaAlO2ÓėHNO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 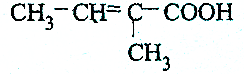 | B£® | 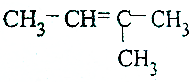 | ||
| C£® | 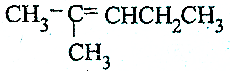 | D£® | H2CØTCH2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £»¼Ó¾Ū·“Ó¦£»
£»¼Ó¾Ū·“Ó¦£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | NH4Al£ØSO4£©2ČÜŅŗÓė¹żĮæNaOHČÜŅŗ·“Ó¦£ŗAl3++4OH-ØTAlO2-+2H2O | |
| B£® | IClČÜÓŚĄäµÄĻ”KOHČÜŅŗÖŠ£ŗICl+2OH-ØTCl-+IO-+H2O | |
| C£® | ÓƶčŠŌµē¼«µć½āCuSO4ČÜŅŗ£ŗ2Cu2++4OH-$\frac{\underline{\;µē½ā\;}}{\;}$2Cu”ż+O2”ü+2H2O | |
| D£® | NaAlO2ČÜŅŗÖŠAlO2-µÄĖ®½ā£ŗAlO2-+2H2OØTAl£ØOH£©3+OH- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
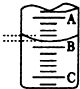 ²ŻĖį£ØH2C2O4£©“ęŌŚÓŚ×ŌČ»½ēµÄÖ²ĪļÖŠ£¬ĘäK1=5.4”Į10-2£¬K2=5.4”Į10-5£¬¾ßÓŠ»¹ŌŠŌ£¬ČÜÓŚĖ®£¬ČÜŅŗÓŠĖįŠŌ£¬ĪŖ²ā¶ØijH2C2O4ČÜŅŗµÄÅØ¶Č£¬Č”øĆČÜŅŗӌ׶ŠĪĘæÖŠ£¬¼ÓČėŹŹĮæĻ”H2SO4ŗó£¬ÓĆÅضČĪŖc mol/L KMnO4±ź×¼ČÜŅŗµĪ¶Ø£®µĪ¶ØŌĄķĪŖ£ŗ2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2”ü+2MnSO4+8H2O
²ŻĖį£ØH2C2O4£©“ęŌŚÓŚ×ŌČ»½ēµÄÖ²ĪļÖŠ£¬ĘäK1=5.4”Į10-2£¬K2=5.4”Į10-5£¬¾ßÓŠ»¹ŌŠŌ£¬ČÜÓŚĖ®£¬ČÜŅŗÓŠĖįŠŌ£¬ĪŖ²ā¶ØijH2C2O4ČÜŅŗµÄÅØ¶Č£¬Č”øĆČÜŅŗӌ׶ŠĪĘæÖŠ£¬¼ÓČėŹŹĮæĻ”H2SO4ŗó£¬ÓĆÅضČĪŖc mol/L KMnO4±ź×¼ČÜŅŗµĪ¶Ø£®µĪ¶ØŌĄķĪŖ£ŗ2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2”ü+2MnSO4+8H2O| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄKMnO4ČÜŅŗĢå»ż/mL | 22.32 | 24.39 | 24.41 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
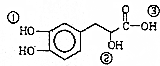
| A£® | øĆÓŠ»śĪļµÄ·Ö×ÓŹ½ĪŖC9H10O5 | |
| B£® | øĆÓŠ»śĪļÄÜ·¢ÉśĖõ¾Ū”¢¼Ó³É”¢ĻūČ„”¢Ńõ»Æ·“Ó¦ | |
| C£® | 1moløĆÓŠ»śĪļ×ī¶ąæÉŅŌŗĶ4molNaOH·¢Éś·“Ó¦ | |
| D£® | øĆÓŠ»śĪļ·Ö×ÓÖŠ¢Ł”¢¢Ś”¢¢Ū3øö-OHµÄĖįŠŌÓÉĒæµ½ČõµÄĖ³ŠņŹĒ¢Ū£¾¢Ł£¾¢Ś |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com