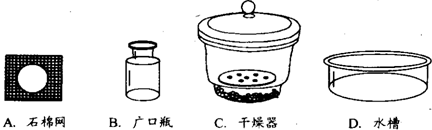
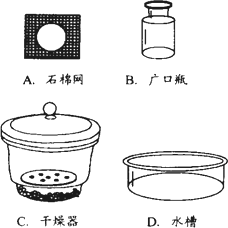
��֪̼��������270�����Ҿ��ֽܷ�Ϊ̼���ơ�ˮ�Ͷ�����̼����̼�������Ȳ��ֽ⣮����ij������������һ��̼�������л���������̼���ƣ�Ϊ�˲ⶨ��Ʒ��̼�����Ƶ���������������ļ��鲽�����£�
��ȡһֻ�ྻ����������������Ϊa g���������м�����Ʒ���Ƶ�������Ϊm
1 g��
�ڼ��ȸ�ʢ����Ʒ��������
�۽����������ȴ������������ʣ������������
�ܶ���ظ�����ں͢������أ��Ƶ�������ʣ������������Ϊm
2 g��
��1��д��̼���������ȷֽ�Ļ�ѧ����ʽ
��
��2����
�����������ƣ������Ⱥ�������ŵ�
�У�����ţ���ȴ��
��3���������⣬��a��m
1��m
2�Ĵ���ʽ��ʾ��Ʒ��̼�����Ƶ���������Ϊ
��
��4������١��ۺܶ͢���Ҫ�õ�����Ϊ0.1g��������ƽ�������������У�m
1-m
2����ֵ����0.6g��������Ʒ��̼�����Ƶ���������Ϊ90%�������������Ʒ���ٿˣ�