
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飺
(һ)����ʽ��ȷ����
(1)���л���A�����������г��ȼ�գ�ʵ���ã�����5.4 g H2O��8.8 g CO2����������6.72 L(��״����)����������и�Ԫ�ص�ԭ�Ӹ�������________��
(2)�������Dzⶨ���л����������Է����������õ���ͼ1��ʾ����ͼ��������Է�������Ϊ________�������ʵķ���ʽ��________��
(3)���ݼۼ����ۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ______________________��
(��)�ṹʽ��ȷ����
(4)�˴Ź��������ܶ��л�������в�ͬλ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ(�ź�)�����ݷ�ֵ(�ź�)����ȷ����������ԭ�ӵ��������Ŀ�����磺���ȼ���(Cl��CH2��O��CH3)��������ԭ����ͼ2�����ⶨ���л���A�ĺ˴Ź�������ʾ��ͼ��ͼ3����A�Ľṹ��ʽΪ________��

ͼ1 ͼ2 ͼ3
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ����NaHCO3��KHCO3��ɵĻ������Ʒ��ij��Ũ�ȵ����ᷴӦ����ʵ�飬����������±���
| ʵ���� | �� | �� | �� |
| �������/mL | 50 | 50 | 50 |
| m(�����)/g | 9.2 | 26.56 | 36.8 |
| V(CO2)(��״��)/L | 2.24 | 4.48 | 4.48 |
�����������ݣ�����˵���в���ȷ���� (����)��
A���ɢ١��ڿ�֪�����е��������
B���ɢڡ��ۿ�֪���������������ӣ����������û�б仯��˵�������Ѿ���Ӧ��ȫ
C��������������ʵ���Ũ��Ϊ0.4 mol��L��1
D���û������NaHCO3�����ʵ�������Ϊ50%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ƿ���Ϊ��������Դ�����³�ѹ�¶�����̼�������Ʒ�Ӧ������������������28 g����Ӧ���й����ʵ���������ȷ����(NA��ʾ�����ӵ�����) ( )
| ������̼ | ̼���� | ת�Ƶĵ��� | |
| A | 1mol | NA | |
| B | 22.4L | 1mol | |
| C | 106 g | 1mol | |
| D | 106g | 2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������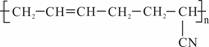 �������������͡��������ܣ�
�������������͡��������ܣ�
�ϳɶ�����ԭ���� �� ��
��CH2=CH��CH=CH2 ��CH3��C��C��CH3 ��CH2=CH��CN
��CH3-CH=CH-CN ��CH3��CH=CH2 ��CH3��CH=CH��CH3
A���٢ۢ� B���٢� C���٢ܢ� D���ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�۲����нṹ��ʽ���ش��������⣺
 |
������Ľṹ��ʽΪ
��1��a��������__________��
��2��c��������________________��

��
��1�����������__________________________��
��2�����л���Ϊϩ���ӳɵIJ����ԭ��ϩ���Ľṹ������_______�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
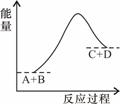
��֪��ӦA+B=C+D�������仯��ͼ��ʾ������˵����ȷ���� �� ��
A���÷�ӦΪ���ȷ�Ӧ
B���÷�ӦΪ���ȷ�Ӧ
C����Ӧ��������������������������
D���÷�Ӧֻ���ڼ��������²��ܽ���
 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��װ���Ҵ����ձ���Ͷ��һС������ƣ����ж�ʵ���������������ȷ���� �� ��
A�����ۻ���С�� B���ƿ�����Ҵ�Һ�������
C���ƿ����Ҵ���Һ�����ζ� D���ƿ���������ݷų���������ը����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�Ԫ�����ʵ�˵������ȷ���� (����)��
A���������е����Ų�ʽ��ԭ���У���1s22s22p63s23p2����1s22s22p3����1s22s22p2����1s22s22p63s23p4��ԭ�Ӱ뾶�����Ǣ�
B���������м۵����Ų�ʽ��ԭ���У���3s23p1����3s23p2
��3s23p3����3s23p4��һ�����������Ǣ�
C����Na��K��Rb����N��P��As����O��S��Se ����Na��P��Cl��Ԫ�صĵ縺����ԭ������������������Ǣ�
D��ijԪ����̬��̬ԭ �ӵ�������(kJ��mol��1)�ֱ�Ϊ738��1 451��7 733��10 540��13 630��17 995��21 703��������������Ӧʱ�������ɵ���������
�ӵ�������(kJ��mol��1)�ֱ�Ϊ738��1 451��7 733��10 540��13 630��17 995��21 703��������������Ӧʱ�������ɵ���������
X3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������������飬�ڱ��飬�������飬�������飬�ݹ����У��е��ɸߵ��͵�˳��������ȷ����(����)
A���٢ڢۢܢ� B���ݢۢܢ٢�
C���ݢ٢ۢܢ� D���ۢܢݢڢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com